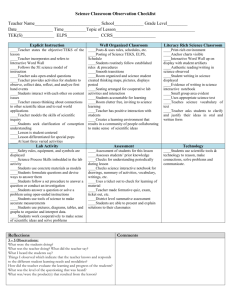
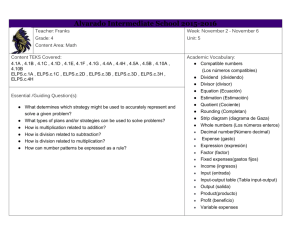
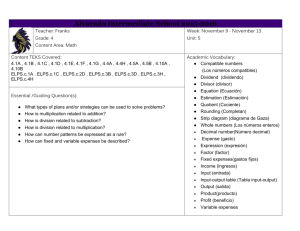
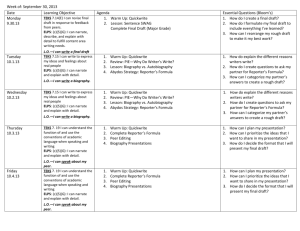
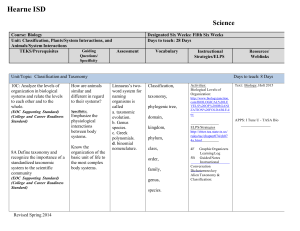
High School Science IPC Unit 02 Exemplar Lesson 01
advertisement

INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 01 TITLE : Unit 01: Laboratory Management SUGGESTED DURATION : 4 days Printer Friendly Version 12_S_IPC_01_IFD.pdf State Resources: Texas Education Agency – Texas Safety Standards. Retrieved from http://www.tea.state.tx.us/index2.aspx?id=5483 (look under Documents). Texas Safety Standards: Kindergarten through Grade 12, 2nd Edition “Science Safety Information and Resources” “Laws and Rules” Exemplar Lessons Rubric(s) for Performance Indicators Unit Test High School Science IPC Unit 01 Exemplar Lesson 01: Safety in the High School Science IPC Unit 01 Rubric 01 Integrated Physics and Chemistry High School Unit 01: Laboratory IPC Laboratory and Creating the Science Notebook High School Science IPC Unit 01 Rubric 02 Management 2012-2013 Instructional Resource(s) Creating the Science Notebook: A Manual for Secondary Teachers Rationale This unit bundles student expectations that address safety and the use of tools and equipment in the integrated physics and chemistry classroom. This unit is designed to give students an overview of safety issues that are specific to the classroom area where they conduct labs. While it is a good idea to introduce and reinforce the importance of safety at the beginning of the course, safety is a concept that should be taught and reinforced every time the students work in the laboratory or field setting. Proper understanding of the availability and use of tools and equipment will also be addressed in this unit. Prior to this unit, students have had opportunities in prior grades to learn safety rules, safe use of equipment, and organization of student work within science notebooks. During this unit, students will demonstrate and use knowledge of safe practices including wearing safety goggles, washing hands, using materials appropriately, as well as use of other preventative equipment such as, aprons, gloves and chemical splash goggles. In addition, students will create science notebooks to collect, record, and analyze information. After this unit, safety is a concept that should be taught and reinforced every time students work in the laboratory or field setting. Notebooking will be utilized throughout the year as well. According to research: Laboratory investigations are essential for the effective teaching and learning of science. A school laboratory investigation (“lab”) is an experience in the laboratory, classroom, or the field that provides students with opportunities to interact directly with natural phenomena or with data collected by others using tools, materials, data collection techniques, and models. (NRC, 2006, p. 3) “Inherent in laboratory-based activities is the potential for injury. Studies show that safety in K–12 school science instruction needs immediate and significant attention” (NSTA, 2010). Science teachers and students must have safety training and access to appropriate safety equipment, such as eye/face wash stations, splash-proof safety goggles, emergency blankets, safety showers and fire extinguishers. Eye/face wash stations should be activated weekly. Risks are reduced and liability can be minimized when these steps are taken. Texas Safety Standards and Science Facilities Standards (two publications of the Texas Education Agency and the Charles A. Dana Center at The University of Texas at Austin), the NSTA Guide to School Science Facilities, and NSTA Exploring Safely: A Guide for Elementary Teachers are excellent resources for laboratory safety and facility requirements. National Research Council (NRC). 2006. America’s lab report: Investigations in high school science. Washington, DC: National Academy Press. National Science Teachers Association. (2010) NSTA position statement: Liability of science educators for laboratory safety. Retrieved April 12, 2010, from http://www.nsta.org/about/positions/liability.aspx. Misconceptions None Identified Performance Indicators Concepts Key Understandings For Learners High School Science Integrated Physics and Chemistry Unit01 PI01 Nature of Science – Safety; Tools and Conducting scientific investigations in a safe manner with considerations for resource conservation increases Within the science notebook, demonstrate knowledge of how to operate Equipment; Communicating the quality of the investigation, decreases risk to the investigator, and lessens negative impact on the Conclusions; Communicating environment. The organization of science activities helps model Data scientific methods, communicates conclusions, and allows for the ability to replicate experimental results. emergency safety equipment, such as a fire extinguisher, fire blanket, and face/eyewash, by explaining in writing how to use the equipment. Additionally, demonstrate knowledge of safe practices, such as wearing safety goggles, washing hands and using materials appropriately, as well as use of other preventative equipment such as, aprons, gloves and chemical splash goggles, by explaining, in writing, the class safety rules for investigations. Standard(s): I.1A , I.2C , I.2E ELPSELPS.c.5B , ELPS.c.5C , ELPS.c.5E , ELPS.c.5F Key Academic Vocabulary Supporting Conceptual Development Hypotheses – tentative and testable statements that must be capable of being supported or not supported by evidence 110327,110327,1 TEKS# SE# I.1 TEKS Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: Unit Level Specificity I.1A demonstrate safe practices during laboratory and field Demonstrate investigations. SAFE PRACTICES DURING FIELD AND LABORATORY INVESTIGATIONS Including, but not limited to: Wear appropriate safety equipment, such as goggles, aprons, and gloves. Know location of safety equipment, such as fire extinguisher, safety shower, and eye wash. Follow classroom guidelines, as outlined in the Texas Education Agency Texas Safety Standards. o o o o o o I.2 Possible examples may include Read or study the science activity or laboratory investigation prior to conducting the investigation. Know and follow all safety rules prior to the investigation. Be alert during the laboratory time. Do not attempt unauthorized activities. If a chemical spill occurs, report it immediately, and follow the instructions of the teacher. o o Keep your area clean. Do not enter preparatory or equipment storage rooms or o chemical storerooms. Always wash your hands for at least 20 seconds with soap and warm water before leaving the laboratory. Use lab equipment appropriately. Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2C collect data and make measurements with precision Collect DATA Including, but not limited to: Observations Measurements Demonstrate use of appropriate equipment to collect data. Make MEASUREMENTS WITH PRECISION Including, but not limited to: Accuracy (how close a measured value is to the actual (true) value) Precision (how close the measured values are to each other) TxCCRS Note: 1. I.2E communicate valid conclusions. Nature of Science – A4 – Rely on reproducible observations of empirical evidence when constructing, analyzing, and evaluating explanations of natural events and processes. Communicate VALID CONCLUSIONS Including, but not limited to: Communicate conclusions in oral, written, and graphic forms. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Use essential vocabulary of the discipline to communicate conclusions. Present scientific information in appropriate formats for various audiences. 10 TEKS# SE# I.1 Scientific Process TEKS: Choose appropriate scientific processes to support your instruction. Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A demonstrate safe practices during laboratory and field investigations I.1B demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section I.2B plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology I.2C collect data and make measurements with precision I.2D organize, analyze, evaluate, make inferences, and predict trends from data I.2E communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A in all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student I.3B communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials I.3C draw inferences based on data related to promotional materials for products and services I.3D evaluate the impact of research on scientific thought, society, and the environment I.3E describe connections between physics and chemistry and future careers I.3F research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# Subsection C: Cross-curricular second language acquisition essential knowledge and skills. ELPS.c.5 Cross-curricular second language acquisition/writing ELPS.c.5 The ELL writes in a variety of forms with increasing accuracy to effectively address a specific purpose and audience in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in writing. In order for the ELL to meet gradelevel learning expectations across foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations do not apply until the student has reached the stage of generating original written text using a standard writing system. The student is expected to: ELPS.c.5B write using newly acquired basic vocabulary and content-based grade-level vocabulary ELPS.c.5C spell familiar English words with increasing accuracy, and employ English spelling patterns and rules with increasing accuracy as more English is acquired ELPS.c.5E employ increasingly complex grammatical structures in content area writing commensurate with grade-level expectations, such as: ELPS.c.5F write using a variety of grade-appropriate sentence lengths, patterns, and connecting words to combine phrases, clauses, and sentences in increasingly accurate ways as more English is acquired High School Science IPC Unit 02 Exemplar Lesson 01: Understanding Elements, Compounds, and the Periodic Table Printer Friendly Version 12_SIPC0201 Lesson Synopsis After exploring the structure of the atom, students will be reintroduced to the periodic table and its usefulness in classifying properties of elements and predicting chemical bonding. This lesson will also include the transition metals as well as the f-block elements (lanthanide and actinide series). TEKS I.6 Science concepts.. The student knows that relationships exist between the structure and properties of matter. The student is expected to: I.6B Relate chemical properties of substances to the arrangement of their atoms or molecules. I.6D Relate the physical and chemical behavior of an element, including bonding and classification, to its placement on the Periodic Table. Scientific Process TEKS: Choose appropriate scientific processes to support your instruction. I.1 Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3F Research and describe the history of physics and chemistry and contributions of scientists. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit02 PI01 Write a summary predicting the likely properties of a provided set of elements. Indicate which elements would likely bond together, and relate this information to each element’s placement on the periodic table. Justify answers using terms such as group, oxidation number, octet rule, ionic compound, and covalent molecule. Standard(s): I.2E , I.6B , I.6C , I.6D ELPSELPS.c.5B , ELPS.c.5G Key Understandings The physical and chemical behavior of an element is related to its placement on the periodic table. — How can the periodic table be used to predict the behavior of elements? The periodic table helps us to determine which elements will bond with others to form compounds. — Will all elements form a bond with every other element? — Are all bonds the same? Vocabulary of Instruction periodic table atomic mass period group family atomic number proton neutron electron valence electron metal nonmetal metalloids transition metals octet rule element compound mixture ionic compound covalent molecule oxidation number matter High School Science IPC Unit 03 Exemplar Lesson 01: States of Matter Printer Friendly Version Exemplar Lesson 01 Lesson Synopsis With this lesson, students begin the transition from learning how matter is organized to exploring some specific properties of matter. The focus for this lesson is the states of matter with an emphasis on the concept that some properties of matter can change even though the original substance remains chemically unchanged. TEKS I.6 Science concepts.. The student knows that relationships exist between the structure and properties of matter. The student is expected to: I.6A Examine differences in physical properties of solids, liquids, and gases as explained by the arrangement and motion of atoms, ions, or molecules of the substances and the strength of the forces of attraction between those particles. Scientific Process TEKS I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit03 PI01 Create a diagram that compares the arrangement of particles to the different states of matter, and give an example of a material (other than water) in all three phases (solid, liquid, and gas). The diagram should include a written description of the arrangement of all three states of matter, relative strength of the force of attraction between the particles of each state of matter, and change in energy involved as the material changes from a solid to a liquid to a gas. Standard(s): I.2D , I.6A , I.7A ELPSELPS.c.1E , ELPS.c.5F , ELPS.c.5G Key Understandings Each state of matter has physical properties that can be explained by the arrangement and motion of particles that make up the substance and by the forces of attraction between the particles. — What are some physical properties that can be explained by the arrangement and motion of the particles in the substance? (density, shape, conductivity, etc.) Changes in the state of matter represent a change in energy and in the arrangement of atoms in a substance. — How does the arrangement of the particles in a substance change when the state of matter changes? Vocabulary of Instruction physical change phase change sublimation melting Materials 400 mL beaker (400 mL, 1 per pair) beaker (50 mL, 1 per pair) beaker (large, 1 per group) Celsius thermometer (1 per pair) chart paper (per group) cups (plastic or paper, several per pair) gloves (heat/cold resistant, per student) graduated cylinder (100 mL, 1 per pair) boiling point freezing point evaporation condensation compound density graph paper (several sheets, per group) hot plate or Bunsen burner (1 per group) ice ice cream or rock salt (80 mL per pair) markers (per group) milk (240 mL per pair) paper towel (several per pair) periodic table of the elements (see Advance Preparation, 1 per student) resealable plastic bag (gallon, freezer quality, 1 per pair) resealable plastic bag (quart, freezer quality, 1 per pair) ring stand (1 per group) safety goggles (1 per student) spoons (several per pair) sugar (45 mL per pair) thermometer (1 per group) thermometer clamp (1 per group) timing device (1 per student) vanilla or chocolate flavoring (2.5 mL per pair) Attachments Handout: Ice Cream Lab (1 per pair of students) Handout: Phase Change Lab (1 per group) Teacher Resource: Phase Change Lab KEY Handout: Analyze the Phase Diagram (1 per student) Teacher Resource: Analyze the Phase Diagram KEY Resources Websites: o IPC in the Movies – Teacher Quality Grant IPC: http://atlantis.coe.uh.edu/texasipc/content.htm o Phet Interactive Simulations: http://phet.colorado.edu/ http://phet.colorado.edu/en/simulation/states-of-matter o o o o How Does a Refrigerator Work: http://www.wisegeek.com/how-does-a-refrigerator-work.htm States of Matter: http://www.chem4kids.com/files/matter_intro.html Differences in Bonding: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond2.html Differences in Bonding: http://www.diffen.com/difference/Covalent_Bonds_vs_Ionic_Bonds o Periodic Table: STAAR Grade 8 (or Chemistry) Science Reference Materials: http://www.tea.state.tx.us/student.assessment/staar/science/ (click on Grade 8 Science or Chemistry Reference Materials) Advance Preparation 1. Prior to Day 1 and Day 4, arrange for access to student computers and Internet. Ensure that all computers to be utilized have Java capability to run the simulation on Day 1. Java script is a free download. Simulation is listed above in Resources and References section. 2. In order to ensure food safety, all equipment that is used to measure ingredients for the ice cream lab should not have been used to measure chemicals prior to the activity. Measuring cups and kitchen equipment should be substituted in lieu of classroom lab ware. 3. Prior to Day 3, if you have not previously had students affix a copy of the Periodic Table of the Elements, download and print copies for each student from the URL listed above in the Resources and References section. 4. Prepare attachments as necessary. Background Information Prior to this unit, in Unit 02, students were introduced to states of matter as a physical property. Properties of matter were introduced as early as Kindergarten and have been revisited in some way every year. The first goal of this lesson is to present the phase change diagram to students and to review the structure of matter in solid, liquid, and gaseous forms. In addition to the proximity and motion of the molecules, their relative attraction will be addressed as well. The second goal of the lesson is to introduce the concept that a substance can undergo a dramatic change, such as changing from a solid to a liquid, without undergoing a chemical change. By emphasizing what is not a chemical change, this unit helps set the stage for Unit 04 – Chemical Reactions where chemical changes do occur. Every student can relate to how water changes from ice (solid), to water (liquid), and then boils to change to steam (gas), but it is important to make sure that students realize that these phase changes occur in substances other than water. Students also need to be aware that other changes, such as sublimation, may occur. For further information on states of matter, please visit the following website: http://www.chem4kids.com/files/matter_intro.html GETTING READY FOR INSTRUCTION SUPPLEMENTAL PLANNING DOCUMENT CSCOPE lessons are one instructional approach for teaching the content and cognitive rigor aligned with the TEKS and the Performance Indicators within the Instructional Focus Document (IFD). Instructors are encouraged to supplement and substitute resources, materials, and activities to differentiate and enhance instruction as appropriate. Original lessons may be created using the Content Creator within the Tools Tab located at the top of the CSCOPE webpage. INSTRUCTIONAL PROCEDURES Instructional ProceduresENGAGE Delete Instructional Procedures ENGAGE Notes for Teacher 1 Day = 50 minutes Suggested Day 1 1. Divide students into groups of 2–3. Instructional Notes: 2. Ask students to recall the three states of matter (solids, liquids, and gases). Inform students that they Test this simulation on your student computers prior to having your students complete this investigation (see Advance Preparation). are going to explore an online simulation that will illustrate or model how molecules behave in each of these three phases. Most Internet computers have Java capability, but if not, you will need to contact district technical support install Java. If Internet 3. 4. You may wish to assign group roles or instruct each group to take turns using the simulation. All students need to record notes in their science notebooks. Instruct groups to go to the following web address and select “Run Now” to run the simulation: http://phet.colorado.edu/en/simulation/states-of-matter bandwidth is an issue, the Phet applications can be pre-installed on each computer. This activity is not designed for students to obtain a particular result or answer, but rather to manipulate and observe something that they otherwise could not see (molecular motion in different phases). Remind students that scientists use models and simulations to help describe and explain what they otherwise could not observe. 5. 6. Instruct the groups to change each of the settings in the “solid, liquid, gas” simulation to see what the various settings do and to look for patterns when the temperature is adjusted. Instruct students to record the temperature changes for each of the four atoms/molecules as the state is changed from solid to liquid to gas. Students should also make notes on the settings and any observations they make or any patterns /trends they notice. 7. When students have had time to explore the simulation, facilitate a class discussion in which students reflect on their observations. Remind students to keep notes in their science notebooks. 8. Ask: Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) Misconceptions: Students may think that only water can melt, boil, and freeze. Science Notebook: Which atom/molecule took the most heat to make it boil and fill the container? Students record data, observations, and discussion notes from simulation in their science notebooks. (Water) 9. Display the chemical formula for water on the board. 10. Say: Water has a strong attractive force between the hydrogen atom and the oxygen atom that hold the atoms close together. This makes it harder to boil water. 11. Continue the discussion using the following as discussion items: Which phase is the densest for neon? (Solid) How were the molecules arranged differently for solids, liquids, and gases? Very close for solids, close together for liquids, far apart for gases How did temperature relate to the phases? Hotter from solids to gases How did the temperature and energy in the system affect the attraction between molecules? Molecules are less attracted to each other as they go from solid to liquid to gas due to increasing energy in the system and distance between molecules. Describe the changes in movement/speed of the molecules as the atoms/molecules changed state. Molecules speed became faster from solid to liquid to gas. How did the energy transfer from one molecule to another? Energy transferred as the molecules vibrated faster and collided with each other. 12. Instruct students to draw detailed sketches of a solid, liquid, and gas in their science notebooks, showing the arrangement of the particles as they observed them in the simulation. EXPLORE – Ice Cream Anyone?Delete EXPLORE – Ice Cream Anyone? 1. Divide students into pairs. 2. Distribute a copy of the Handout: Ice Cream Lab to each pair of students. 3. Instruct students to read the instructions on the investigation handout. Answer any questions they Suggested Day 2 Materials: milk (240 mL per pair) sugar (45 mL per pair) may have. Remind students to remain at their station to complete the investigation. 4. 5. 6. Monitor to ensure food safety and assist students as necessary. After students have completed the investigation, ask student to reflect on their findings. Ask students to report on the phase change that has occurred. (Liquid to solid) Ask students to think about what could be happening on a molecular level to cause the ingredients to become solid. Accept all answers. 7. Guide students to reflect on the change in energy and the resulting changes in the molecules’ properties as the materials cooled. Freezing is the process that causes a substance to change from a liquid to a solid. Freezing occurs when the molecules of a liquid slow down enough that their attractions cause them to arrange themselves into fixed positions as a solid. cups (plastic or paper, several per pair) ice cream or rock salt (80 mL per pair) vanilla or chocolate flavoring (2.5 mL per pair) beaker (50 mL, 1 per pair) graduated cylinder (100 mL, 1 per pair) ice (per pair) 400 mL beaker (400 mL, 1 per pair) resealable plastic bag (gallon, freezer quality, 1 per pair) gloves (heat/cold resistant, per student) resealable plastic bag (quart, freezer quality, 1 per pair) paper towel (several per pair) spoons (several per pair) Celsius thermometer (1 per pair) Attachments: 8. Ask: Handout: Ice Cream Lab (1 per pair) What are some physical properties that can be explained by the arrangement and motion of the particles in the substance? (density, shape, conductivity, etc.) Safety Note: 9. Ask students to work with their partner to describe the phase change in their science notebooks and, additionally, to describe what was occurring on molecular level as the ingredients cooled. In order to ensure food safety, all equipment that is used to measure ingredients for the ice cream lab should not have been used to measure chemicals prior to the activity. Measuring cups and kitchen equipment should be substituted in lieu of classroom lab ware. Instructional Notes: Provide the students a location such as a bucket or cooler to dump their used ice water so that rock salt does not clog your lab drains. EXPLORE/EXPLAIN – Phase Change LabDelete EXPLORE/EXPLAIN – Phase Change Lab Suggested Day 3 1. Divide students into groups of 2–3. 2. Explain that groups are going to conduct a simple investigation to explore what occurs when substances change from one phase to another. 3. Distribute a copy of the Handout: Phase Change Lab to each group. 4. Instruct students to read the instructions on the investigation handout. Answer any questions they may have. Do not allow students to add heat to their set ups until you have approved their materials set up for safety. 5. Allow each group time to complete the investigation while actively monitoring to ensure each group is following appropriate safety rules. 6. Materials: After students have completed the activity, ask each group to share or post their results. thermometer (1 per group) thermometer clamp (1 per group) ring stand (1 per group) hot plate or Bunsen burner (1 per group) beaker (large, 1 per group) ice (per group) graph paper (several sheets, per group) safety goggles (1 per student) timing device (1 per student) periodic table of the elements (see Advance Preparation, 1 per student) Ask students to reflect on any differences in results. (See the Instructional Notes.) Attachments: Ask: What do we call the temperature at which a substance melts? (Melting point) What do we call the temperature at which a substance boils? (Boiling 7. point) What is the phase change called when a substance changes directly from a solid to a gas, without first becoming a liquid? (Sublimation) Continue the discussion, using questions from the Teacher Resource: Analyze the Phase Change Diagram KEY to guide the discussion. These questions will be also be used during the Evaluation phase of the lesson. 8. 9. Ask students to record the terms melting point, boiling point, and sublimation and their definitions in their science notebooks. When students have finished their handouts, they should affix them in their notebooks and prepare to answer questions. Handout: Phase Change Lab (per group) Teacher Resource: Phase Change Lab KEY Teacher Resource: Analyze the Phase Diagram KEY Safety Note: Safety should be emphasized throughout the investigation. While the materials are not hazardous, the hot plate/Bunsen burner and boiling water present a burn hazard. Instructional Notes: In order to expedite set up, you may wish to set up a model lab station with the hot plate, ring stand, beaker, thermometer, and clamp. Each group may come up with a slightly different temperature for melting or boiling point. This is an opportunity for students to reflect on variability in investigations. Possible variations may occur due to poorly calibrated thermometers or contaminates in the water (chlorine, salts, etc.). Use questions from the Teacher Resource: Analyze the Phase Change Diagram KEY to guide the student discussion after the investigation. 10. Facilitate a class discussion in which students reflect on the following questions: Possible websites to simplify the differences in bonding may be found at the following URLs: What are the three types of bonding we learned about in the previous unit? Covalent boding is the bonding of two nonmetals, ionic bonding is the bonding of a nonmetal and a metal, and metallic bonding is the bonding found http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond2.html http://www.diffen.com/difference/Covalent_Bonds_vs_Ionic_Bonds in metals. What is the order of strength of these types of bonds from strongest to weakest? Ionic, covalent, metallic How do you think that strength might affect melting points? Ionic bonds Misconceptions: are the strongest so they will have higher melting points. Students may think that materials can only have properties of one state of matter. 11. Ask students to predict which of the following compounds will have the highest melting point. Help them use the periodic table to decide which are metals or nonmetals. Find other examples if needed. Water or salt? Salt is ionic and has a MP of 801ºC Napthalene C10H8 or Magnesium oxide MgO? Magnesium oxide is ionic and has MP of 2800ºC. Napthalene (in moth balls) has a MP of 80ºC. ELABORATE – RefrigerationDelete ELABORATE – Refrigeration 1. Divide students up into groups of 2–3. 2. Inform students that today the class is going to explore a common application of phase changes and the energy (heat) absorbed and released as a result of those changes. 3. Instruct each group to research how refrigerators work. Explain to students that they will need to read carefully, so they begin to understand how this appliance works. There are many sites available Suggested Day 4 Materials: markers (per group) chart paper (per group) to choose from, but each group should find a site similar to: http://www.wisegeek.com/how-does-a-refrigerator-work.htm Instructional Notes: Students will also need to look for images that illustrate how refrigerators work as well. They may need to use the search string “refrigerator schematic image”, “refrigerator system image” or Internet. Students should be able to find all of the information they need in less than twenty (20) minutes. Monitor groups to make sure This topic (how a refrigerator works) is very easy to find on the they are looking for general information and using time wisely. “refrigerator system cycle image”. 4. Monitor student groups to guide them in finding simple graphics that illustrate basic components. 5. Distribute a piece of chart paper to each group. 6. Using the images the groups found, each group should sketch and label a diagram on their chart paper. Sketches should show the flow of heat and energy in a typical refrigerator system. 7. Each member of the group should draw a smaller version of their diagram with labels and a short explanation as to what is happening in their science notebooks. Remind students to add labels to their sketches. 8. When each group has had enough time to complete their assignment, ask a group to volunteer to explain how a refrigerator works. Ask students to compare the different groups’ diagrams. Ask: Does the shape of path of the coils make a difference in how the refrigerator works? (No EVALUATE – Performance IndicatorDelete EVALUATE – Performance Indicator Performance Indicator Create a diagram that compares the arrangement of particles to the different states of matter, and give an example of a material (other than water) in all three phases (solid, liquid, and gas). The diagram should include a written description of the arrangement of all three states of matter, relative strength of the force of attraction between the particles of each state of matter, and change in energy involved as the material changes from a solid to a liquid to a gas. (I.2D; I.6A; I.7A) 1E; 5F, 5G Suggested Day 5 1. Distribute a copy of the Handout: Analyze the Phase Diagram to each student. 2. Explain that each student on their own should complete the handout sketching the molecules of a solid, liquid, or gas within the drawn containers. After completing their 3. sketches, students should answer the follow-up questions. After everyone has had time to complete the activity, facilitate a discussion on the correct 4. answers and sketches with the class. Instruct students to affix their completed handout into their science notebooks or collect for grading. High School Science IPC Unit 04 Exemplar Lesson 01: Conservation of Mass Printer Friendly Version 12_SIPC0401.pdf Lesson Synopsis In this lesson, students will explore the concept that mass is conserved when substances undergo chemical change and that the number and kind of atoms are the same in the reactants and products. In addition, they will recognize valence electrons and that the behavior of valence electrons is responsible for chemical changes. Students will plan and implement an investigation to demonstrate their understanding of the law of conservation of mass. TEKS I.7 I.7B I.7C Science concepts.. The student knows that changes in matter affect everyday life. The student is expected to: Recognize that chemical changes can occur when substances react to form different substances and that these interactions are largely determined by the valence electrons. Demonstrate that mass is conserved when substances undergo chemical change and that the number and kind of atoms are the same in the reactants and products. Scientific Process TEKS I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2E Communicate valid conclusions. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit04 PI01 Select a balanced equation from a laboratory investigation. Use the balanced equation to explain and demonstrate the law of conservation of mass. Present information in a diagram, and explain, in writing, how the balanced equation represents a chemical change. Demonstrate that the number and kind of atoms are the same in the reactants and products. Standard(s): I.2E , I.7C ELPSELPS.c.1C , ELPS.c.1E , ELPS.c.5G Key Understandings Matter and mass are conserved in chemical reactions. — In what ways is it helpful to know that the mass before and after a reaction is constant? — What information can a chemical equation relay? The law of conservation of mass applies only to a closed system. — What is a closed system? — Why does the law of conservation of mass apply only to closed systems? — How does the law of conservation of mass apply to chemical reactions? Vocabulary of Instruction law of conservation of mass coefficient chemical equation yield sign reactants products subscript valence electrons High School Science IPC Unit 05 Exemplar Lesson 01: Environmental Impact of Chemical Reactions Printer Friendly Version 12_SIPC0501.pdf Lesson Synopsis In this lesson, students research and describe the environmental and economic impact of the end-products of chemical reactions. Students conduct classroom and field investigations to gain insight into the effects of natural processes and human activity on the quality of our air and water. Investigations include simulations of acid rain and the greenhouse effect, as well as water quality testing. Both positive and negative impacts are explored. Students describe how the effects on our environment result in economic impacts. TEKS I.7 Science concepts.. The student knows that changes in matter affect everyday life. The student is expected to: I.7F Research and describe the environmental and economic impact of the end-products of chemical reactions such as those that may result in acid rain, degradation of water and air quality, and ozone depletion. !!Missing Resource (Class.Key) :: Portal.Resources.Csp.Resources.ProcessTeksScientificView I.1 Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. High School Science IPC Unit 06 Exemplar Lesson 01: The Nature of Solubility Printer Friendly Version 12_SIPC0601.pdf Lesson Synopsis This lesson will be the first of a two lessons on the nature of solubility. Topics addressed include defining the terms solubility, solvent, and solute. Students will analyze the effect of particle size, temperature, and pressure on the rate of solubility. In addition, students will learn about some of the properties that lead to water being considered a universal solvent. TEKS I.6 Science concepts.. The student knows that relationships exist between the structure and properties of matter. The student is expected to: I.6E Relate the structure of water to its function as a solvent and investigate the properties of solutions and factors affecting gas and solid solubility, including nature of solute, temperature, pressure, pH, and concentration. Scientific Process TEKS: Choose appropriate scientific processes to support your instruction. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit06 PI01 Analyze the effects of various factors on solubility by interpreting data obtained through classroom experiments and hypothetical data. Complete a laboratory report including graph(s) to organize and communicate the data and conclusions. Within the report, include a discussion on the relationship of the structure of water to its function as a solvent. Standard(s): I.2C , I.2D , I.2E , I.6E ELPSELPS.c.1C , ELPS.c.4I , ELPS.c.5F Key Understandings The structure of water molecules makes it a good solvent. — In what ways does the structure of water make it a good solvent? Water is considered the universal solvent because of its structure. — In what ways is water a good solvent and thus, considered the universal solvent? Different properties of solutions and factors affect solubility rates. — What are the properties that affect solid solubility rates? — What are the properties that affect gas solubility rates? Vocabulary of Instruction solubility solvent solute solutions structure polar molecule universal solvent pressure temperature dilute High School Science IPC Unit 07 Exemplar Lesson 01: Describing and Calculating Motion Printer Friendly Version 12_SIPC0701.pdf Lesson Synopsis This lesson reviews the concepts of speed, velocity, and acceleration with an emphasis on both calculations and the interpretation of tables and graphs. TEKS I.4 Science concepts.. The student knows concepts of force and motion evident in everyday life. The student is expected to: I.4A Describe and calculate an object's motion in terms of position, displacement, speed, and acceleration. I.4B Measure and graph distance and speed as a function of time using moving toys. Scientific Process TEKS I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit 07 PI 01 Create a visual display, such as a comic strip, using graphs, formulas, and/or charts to predict the future position of an object in motion at a certain time, and then demonstrate the prediction and outcome to the class. Standard(s): I.2D , I.2E , I.4A , I.4B ELPSELPS.c.1C , ELPS.c.1E , ELPS.c.3D , ELPS.c.3H , ELPS.c.5B Key Understandings An object’s future position can be predicted with great accuracy if certain variables are known. — What information must we know in order to predict an object’s future position? Vocabulary of Instruction motion position displacement distance time speed velocity acceleration High School Science IPC Unit 08 Exemplar Lesson 01: Traditional Forces and Motion Printer Friendly Version 12_SIPC0801.pdf Lesson Synopsis Students will investigate forces, Newton’s laws and momentum, as the primary focus is to help students understand the qualitative relationship of forces. Students will build on the experiences, performances and projects in the previous units of measuring and graphing of motion. This will allow students to see that these topics are related and intertwined, not just stand alone concepts. TEKS I.4 Science concepts.. The student knows concepts of force and motion evident in everyday life. The student is expected to: I.4C Investigate how an object's motion changes only when a net force is applied, including activities and equipment such as toy cars, vehicle restraints, sports activities, and classroom objects. I.4D Assess the relationship between force, mass, and acceleration, noting the relationship is independent of the nature of the force, using equipment such as dynamic carts, moving toys, vehicles, and falling objects. I.4E Apply the concept of conservation of momentum using action and reaction forces such as students on skateboards. Scientific Process TEKS I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3F Research and describe the history of physics and chemistry and contributions of scientists. GETTING READY FOR INSTRUCTION Performance Indicators High School Science Integrated Physics and Chemistry Unit 08 PI 01 Create a booklet that includes a distance-time graph and velocity-time graph from the data collected from a motion investigation with toy cars. Include a paragraph explaining each graph and the measurements needed to calculate the speed, acceleration, and momentum of the cars. Show calculations where necessary. In addition, calculate the momentum of the cars at three different points along the distance-time graph. Mark these areas on the graph. Standard(s): I.2C , I.2D , I.2E , I.4C , I.4D , I.4E ELPSELPS.c.1C , ELPS.c.5B , ELPS.c.5G Key Understandings Only unbalanced forces can cause motion. — What causes an object to experience motion or change motion? — How does Newton’s first law relate to the motion of an object? — What is inertia? The motion of an object depends on the relationship between force, mass, and acceleration. — How does Newton’s second law relate to the motion of an object? — What happens to an object if you apply an unbalanced force to it? In any collision, momentum is conserved. — How does conservation of momentum relate to Newton’s third law? Momentum is a combination of mass and velocity. — How does an object change momentum? — How is an object’s momentum determined? Vocabulary of Instruction force applied unbalanced forces weight motion mass acceleration conservation net force momentum action reaction INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 09 TITLE : Unit 09: Energy: Potential and Kinetic SUGGESTED DURATION : 10 days Printer Friendly Version 12_S_IPC_09_IFD.pdf State Resources: None identified. Unit Test Science IPC Unit 09: Energy: Potential and Kinetic 2012-2013 Rationale This unit bundles student expectations that relate to potential and kinetic energy. Prior to this unit, in Grade 6, students were introduced to kinetic and potential energy and made the connection between energy and force and motion. During this unit, students learn to recognize and demonstrate common forms of kinetic and potential energy. Students also investigate the law of conservation of energy. In the following unit, students will continue the focus on energy through a study of waves. According to the American Association for the Advancement of Science (AAAS) in the Benchmarks for Science Literacy (Project 2061) [online version], “by the end of the 12th grade, students should know that “Science is based on the assumption that the universe is a vast single system in which the basic rules are everywhere the same and that the things and events in the universe occur in consistent patterns that are comprehensible through careful, systematic study.” American Association for the Advancement of Science. (1993). Benchmarks on-line. Retrieved April 25, 2008, from http://www.project2061.org/publications/bsl/online/bolintro.htm. Misconceptions Students may think energy is confined to some particular origin, such as energy from food or energy from the electricity company. Students my think energy is lost in many energy transformations. Students may wonder if energy is conserved, why we are running out of it. Students may think an object at rest has no energy. Students may think the only type of potential energy is gravitational. Performance Indicators Concepts Key Understandings For Learners High School Science Integrated Physics and Chemistry Unit 09 PI 01 Properties – Energy Models – Energy Objects and substances in motion have kinetic energy. Given a specific example, create a chart that shows energy’s journey from potential to kinetic. In a written summary, explain the chart, and describe how the energy was Nature of Science Potential energy comes in different forms. Energy is conserved in a closed system. ultimately conserved. Standard(s): I.2E , I.5A , I.5B , I.5D ELPSELPS.c.1C , ELPS.c.2E , ELPS.c.3D , ELPS.c.4D , ELPS.c.5B Key Academic Vocabulary Supporting Conceptual Development Law of conservation of energy – states the total amount of energy in any closed system remains constant, but may change from one form to another Potential energy – stored energy; the ability of a system to do work due to its position or internal structure Kinetic energy – the mechanical energy that a body has by virtue of its motion The phase 2 College Readiness English Language Arts and Reading vertical alignment team found that the College Readiness Standards in English Language Arts and Reading are well aligned with the Texas Essential Knowledge and Skills. 110327,118094,1 TEKS# SE# I.2 TEKS Unit Level Specificity Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2E communicate valid conclusions. Communicate VALID CONCLUSIONS Including, but not limited to: Communicate conclusions in oral, written, and graphic forms. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Present scientific information in appropriate formats for various Use essential vocabulary of the discipline to communicate conclusions. audiences. I.5 Science concepts.. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to: I.5A recognize and demonstrate that objects and substances in motion Recognize, Demonstrate have kinetic energy such as vibration of atoms, water flowing down a stream moving pebbles, and bowling balls knocking down pins THAT OBJECTS AND SUBSTANCES IN MOTION HAVE KINETIC ENERGY Including, but not limited to: Energy o Kinetic energy Vibration of atoms Water flowing down a stream moving pebbles Bowling balls knocking down pins TxCCRS Physics TxCCRS Understand potential and kinetic energy. I.5B demonstrate common forms of potential energy, including gravitational, elastic, and chemical, such as a ball on an inclined Demonstrate COMMON FORMS OF POTENTIAL ENERGY plane, springs, and batteries Including, but not limited to: Potential o Gravitational o Elastic o TxCCRS Physics TxCCRS Understand potential and kinetic energy. I.5D investigate the law of conservation of energy A ball on an inclined plane Springs Rubber bands Chemical Batteries Investigate THE LAW OF CONSERVATION OF ENERGY Including, but not limited to: Gravitational potential energy Kinetic energy Conversion between o KE = kinetic energy o GPE = gravitational potential energy o How kinetic and potential energy relate to ME(mechanical energy) Mechanical energy = kinetic energy = potential energy ME = PE + KE Calculate the kinetic energy of an object given its mass and velocity. o o Kinetic energy = ½(mass)(velocity)2 KE = 1/2mv2 Calculate the gravitational potential energy of an object given its mass and its height. o Gravitational potential energy = (mass)(acceleration o Introduce chemical potential energy. Analyze energy transformation. o TxCCRS Chemistry TxCCRS Understand the Law of Conservation of Energy and processes of due to gravity)(height) PEg = mgh Potential to kinetic heat transfer. TxCCRS Physics TxCCRS Understand conservation of energy. 18 TEKS# SE# I.1 Scientific Process TEKS: Choose appropriate scientific processes to support your instruction. Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A Know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section. I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. I.3B Communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials. I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# ELPS.c.1 Subsection C: Cross-curricular second language acquisition essential knowledge and skills. The ELL uses language learning strategies to develop an awareness of his or her own learning processes in all content areas. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.1C use strategic learning techniques such as concept mapping, drawing, memorizing, comparing, contrasting, and reviewing to acquire basic and grade-level vocabulary ELPS.c.2 The ELL listens to a variety of speakers including teachers, peers, and electronic media to gain an increasing level of comprehension of newly acquired language in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in listening. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.2E use visual, contextual, and linguistic support to enhance and confirm understanding of increasingly complex and elaborated spoken language ELPS.c.3 The ELL speaks in a variety of modes for a variety of purposes with an awareness of different language registers (formal/informal) using vocabulary with increasing fluency and accuracy in language arts and all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in speaking. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.3D speak using grade-level content area vocabulary in context to internalize new English words and build academic language proficiency ELPS.c.4 The ELL reads a variety of texts for a variety of purposes with an increasing level of comprehension in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in reading. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations apply to text read aloud for students not yet at the stage of decoding written text. The student is expected to: ELPS.c.4D use prereading supports such as graphic organizers, illustrations, and pretaught topic-related vocabulary and other prereading activities to enhance comprehension of written text ELPS.c.5 The ELL writes in a variety of forms with increasing accuracy to effectively address a specific purpose and audience in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in writing. In order for the ELL to meet gradelevel learning expectations across foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations do not apply until the student has reached the stage of generating original written text using a standard writing system. The student is expected to: ELPS.c.5B write using newly acquired basic vocabulary and content-based grade-level vocabulary INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 10 TITLE : Unit 10: Energy: Waves SUGGESTED DURATION : 14 days Printer Friendly Version 12_S_IPC_10_IFD.pdf State Resources: None identified. Unit Test Science IPC Unit 10: Energy: Waves 2012-2013 Rationale This unit bundles student expectations that address concepts relating to waves. Prior to this unit, in Grade 8, students learned how different wavelengths of the electromagnetic spectrum are used to gain information about distances and properties of components in the universe. Students may not have an understanding of frequency and amplitude in waves and the relationship of waves to energy. During this unit, students will study the characteristics and behaviors of energy transferred by waves. After this unit, students will not study waves again until Physics. Misconceptions Students may think you can see and hear a distinct event at the same moment. Students may think sounds can travel through empty space (a vacuum). Students may think sounds cannot travel through liquids and solids. Performance Indicators High School Science Integrated Physics and Chemistry Unit 10 PI 01 Using everyday objects, explore various wave types and their characteristics and behaviors. Create a graphic organizer, such as a layered book, to illustrate and describe the Concepts Key Understandings For Learners Patterns – Waves Waves have specific characteristics, Nature of Science which can be modeled and measured. Waves have many interactions. Energy can be transferred by waves. characteristics and behaviors for of the examples. Standard(s): I.2E , I.5G ELPSELPS.c.1C , ELPS.c.3D , ELPS.c.3H Key Academic Vocabulary Supporting Conceptual Development Transverse wave – oscillations (vibrations of the wave) are perpendicular to direction of the waves (string, water) Longitudinal wave – oscillations are in the same direction as the wave (slinky, sound waves) The phase 2 College Readiness English Language Arts and Reading vertical alignment team found that the College Readiness Standards in English Language Arts and Reading are well aligned with the Texas Essential Knowledge and Skills. 110327,118094,1 TEKS# SE# TEKS I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2E communicate valid conclusions. Unit Level Specificity Communicate VALID CONCLUSIONS Including, but not limited to: I.5 Communicate conclusions in oral, written, and graphic forms. Use essential vocabulary of the discipline to communicate conclusions. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Present scientific information in appropriate formats for various audiences. Science concepts.. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to: I.5G explore the characteristics and behaviors of energy transferred by waves, including acoustic, seismic, light, and waves on water as they superpose on one another, bend Explore THE CHARACTERISTICS AND BEHAVIORS OF around corners, reflect off surfaces, are absorbed by materials, and change direction when entering new materials ENERGY TRANSFERRED BY WAVES Including, but not limited to: Types of waves o Transverse o Longitudinal (compression) Examples of waves o o o o Physics TxCCRS Understand the difference between transverse and longitudinal waves. TxCCRS Understand wave terminology: wavelength, period, frequency, and amplitude. TxCCRS Understand the properties and behavior of sound waves. TxCCRS Know the electromagnetic spectrum. TxCCRS Understand the wave/particle duality of light. Amplitude Wavelength Period Crest Trough Classification of electromagnetic waves Calculate o Velocity = (frequency)(wavelength) o v=fג o Period T = 1/f o Wavelength = ג Behaviors of waves o o o o o TxCCRS Light Water Characteristics of waves o Frequency (hertz) o o o o o Acoustic Seismic Superpose on one another Bend around corners Reflect off surfaces Absorbed by materials Change direction 12 TEKS# SE# Scientific Process TEKS: Choose appropriate scientific processes to support your instruction. I.1 Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A Know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section. I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. I.3B Communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials. I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# ELPS.c.1 Subsection C: Cross-curricular second language acquisition essential knowledge and skills. The ELL uses language learning strategies to develop an awareness of his or her own learning processes in all content areas. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.1C use strategic learning techniques such as concept mapping, drawing, memorizing, comparing, contrasting, and reviewing to acquire basic and grade-level vocabulary ELPS.c.3 The ELL speaks in a variety of modes for a variety of purposes with an awareness of different language registers (formal/informal) using vocabulary with increasing fluency and accuracy in language arts and all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in speaking. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.3D speak using grade-level content area vocabulary in context to internalize new English words and build academic language proficiency ELPS.c.3H narrate, describe, and explain with increasing specificity and detail as more English is acquired INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 11 TITLE : Unit 11: Energy: Electricity SUGGESTED DURATION : 10 days Printer Friendly Version 12_S_IPC_11_IFD.pdf State Resources: None Unit Test Science IPC Unit 11: Energy: Electricity 2012-2013 Rationale This unit addresses student expectations related to electricity, the relationship between electricity and magnetism, and how electricity moves through circuits. Prior to this unit, in Grade 6, students studied energy transformations between chemical and electrical energy. In Grade 8, students explored how different wavelengths of the electromagnetic spectrum, such as light and radio waves, are used to gain information about distances and properties of components in the universe. During this unit, students explore the differences between series and parallel circuits and explore electromagnets. After this unit, students continue the study of energy through the concepts of conversions and conservation. According to the American Association for the Advancement of Science (AAAS) in the Benchmarks for Science Literacy (Project 2061) [online version], “by the end of the 12th grade, students should know that “Investigations are conducted for different reasons, including to explore new phenomena, to check on previous results, to test how well a theory predicts, and to compare theories.” Electricity provides the opportunity to make many predictions before taking measurements either using simulations or real instruments. Students should realize that future results can be predicted with great accuracy before experimentation begins, if the underlying theory or law is valid. American Association for the Advancement of Science. (1993). Benchmarks on-line. Retrieved April 25, 2008, from http://www.project2061.org/publications/bsl/online/bolintro.htm. Misconceptions MISCONCEPTIONS: Students may think current flows around a complete circuit, but it is used by objects like light bulbs so less current returns than leaves the source of the electricity. Students may think current flows from a battery (or other source of electricity) to a light bulb (or other item that consumes electricity), but not from the light bulb back to the battery. Students may think electricity is produced in the wall socket. Students may think all wires are insulated. Performance Indicators High School Science Integrated Physics and Chemistry Unit 11 PI 01 Concepts Key Understandings For Learners Systems – Cycles; Matter Different materials have the ability to act as conductors or insulators, to allow or block the flow of electricity. Regardless of the type of circuit, parallel Create models (virtual or physical) of both series and parallel circuits including a power source, switch, and at least one light per electrical path. Use various materials to demonstrate the circuit can be broken or maintained depending on the conductive properties of the material. Describe or series, electricity in a circuit must follow a closed circular path. the flow of electrons as they flow through the circuits. Standard(s): I.2E , I.5F ELPSELPS.c.1C , ELPS.c.2I , ELPS.c.3D High School Science Integrated Physics and Chemistry Unit 11 PI 02 Systems – Cycles; Matter Electric and magnetic fields are interconnected forces. Using a real-world application, analyze and compare the relationship between electricity and magnetism. Create a poster to demonstrate how electricity and magnetism work together to help the application perform its function. Include appropriate labeled drawings and descriptions. Standard(s): I.2E , I.5C ELPSELPS.c.1C , ELPS.c.5B , ELPS.c.5G Key Academic Vocabulary Supporting Conceptual Development Circuit – a closed conducting circle or loop through which current can flow Conductor – a substance or object that allows electricity to flow through it with low resistance Insulator – a substance or object that does not conduct electricity Electromagnet – an iron or steel core that is magnetized by electric current in a coil that surrounds it 110327,118083,1 TEKS# SE# I.2 TEKS Unit Level Specificity Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of Know THE DEFINITION OF SCIENCE this section Including, but not limited to: Science, as defined by the National Academy of Sciences, is the "use of evidence to construct testable explanations and predictions of natural phenomena, as well as the knowledge generated through this process". Understand SCIENCE HAS LIMITATIONS Including, but not limited to: I.2E communicate valid conclusions. “... some questions are outside the realm of science because they deal with phenomena that are not scientifically testable.” Scientific inquiry may be limited by current technology. Communicate VALID CONCLUSIONS Including, but not limited to: I.3 Communicate conclusions in oral, written, and graphic forms. Use essential vocabulary of the discipline to communicate conclusions. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Present scientific information in appropriate formats for various audiences. Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3E describe connections between physics and chemistry and future careers Describe CONNECTIONS BETWEEN PHYSICS AND CHEMISTRY AND FUTURE CAREERS Including, but not limited to: I.5 Science concepts.. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to: How physics and chemistry are used in various careers I.5C demonstrate that moving electric charges produce Demonstrate magnetic forces and moving magnets produce electric forces THAT MOVING ELECTRIC CHARGES PRODUCE MAGNETIC FORCES AND MOVING MAGNETS PRODUCE ELECTRIC FORCES Including, but not limited to: Electricity and magnetism are two aspects of a single electromagnetic force. o Moving electric charges produce magnetic force. o Moving magnets produce electric forces. Electric currentmagnetic field Describe how a compass uses magnetic fields in relationship to the Earth’s polarity. Describe an electric field. Identify the role of o o Transformers Generators TxCCRS Note: VIII. Physics – I1 – Discuss electric charge and electric force. VIII. Physics – I7 – Understand magnetic fields and their relationship to electricity. I.5F evaluate the transfer of electrical energy in series and parallel circuits and conductive materials Evaluate THE TRANSFER OF ELECTRICAL ENERGY Including, but not limited to: Series circuits Parallel circuits Describe the difference in open and closed circuits. Build series and parallel circuits. Ohm’s law Calculate resistance using voltage and current. o o Identify the symbols in a schematic diagram. o Battery o o o Current = voltage/resistance I = V/R Switch Resistor/amp Wires Describe the flow of electrons as they travel through a circuit. Insulating materials Conductive materials o Investigate the electrical and thermal conductivity of a variety of materials. TxCCRS Note: VIII. Physics – I2 – Gain qualitative understandings of voltage, current, and resistance. VIII. Physics – I5 – Discuss basic DC circuits that include voltage sources and combinations of resistors. VIII. Physics – I6 – Discuss basic DC circuits that include voltage sources and combinations of capacitors. VIII. Physics – I8 – Relate electricity and magnetism to everyday life. 17 TEKS# SE# Process TEKS I.1 Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A Know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section. I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. I.3B Communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials. I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# Subsection C: Cross-curricular second language acquisition essential knowledge and skills. ELPS.c.1 Cross-curricular second language acquisition/learning strategies ELPS.c.1 The ELL uses language learning strategies to develop an awareness of his or her own learning processes in all content areas. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.1C use strategic learning techniques such as concept mapping, drawing, memorizing, comparing, contrasting, and reviewing to acquire basic and grade-level vocabulary ELPS.c.2 Cross-curricular second language acquisition/listening ELPS.c.2 The ELL listens to a variety of speakers including teachers, peers, and electronic media to gain an increasing level of comprehension of newly acquired language in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in listening. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.2I demonstrate listening comprehension of increasingly complex spoken English by following directions, retelling or summarizing spoken messages, responding to questions and requests, collaborating with peers, and taking notes commensurate with content and grade-level needs. ELPS.c.3 Cross-curricular second language acquisition/speaking ELPS.c.3 The ELL speaks in a variety of modes for a variety of purposes with an awareness of different language registers (formal/informal) using vocabulary with increasing fluency and accuracy in language arts and all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in speaking. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.3D speak using grade-level content area vocabulary in context to internalize new English words and build academic language proficiency ELPS.c.5 Cross-curricular second language acquisition/writing ELPS.c.5 The ELL writes in a variety of forms with increasing accuracy to effectively address a specific purpose and audience in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in writing. In order for the ELL to meet gradelevel learning expectations across foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations do not apply until the student has reached the stage of generating original written text using a standard writing system. The student is expected to: ELPS.c.5B write using newly acquired basic vocabulary and content-based grade-level vocabulary ELPS.c.5G narrate, describe, and explain with increasing specificity and detail to fulfill content area writing needs as more English is acquired. INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 12 TITLE : Unit 12: Energy: Conversions and Conservation SUGGESTED DURATION : 10 days Printer Friendly Version 12_S_IPC_12_IFD.pdf State Resources: None Rationale This unit bundles student expectations that examine thermal energy transfer and the law of conservation of energy. Prior to this unit, in Grade 6, students were introduced to the law of conservation of energy and investigated methods of thermal energy transfer, including convection, conduction, and radiation. During this unit, students investigate and demonstrate the movement of thermal energy through solids, liquids, and gases by convection, conduction, and radiation, such as in weather, living, and mechanical systems. They also apply the law of conservation of energy to thermal energy transfer. After this unit, societal impacts for energy usage. After this unit, students explore the societal impacts of the relationships of energy resources. According to the American Association for the Advancement of Science (AAAS) in the Benchmarks for Science Literacy (Project 2061) [online version], “thermal energy in a system is associated with the disordered motions of its atoms or molecules. Gravitational energy is associated with the separation of mutually attracting masses. Electrical potential energy is associated with the separation of mutually attracting or repelling charges. 4E/H7** (BSL) As energy spreads out, whether by conduction, convection, or radiation, the total amount of energy stays the same. However, since it is spread out, less can be done with it. 4E/H3** American Association for the Advancement of Science. (1993). Benchmarks on-line. Retrieved May 08, 2010, from http://www.project2061.org/publications/bsl/online/bolintro.htm. Misconceptions MISCONCEPTIONS: Students may think all liquids boil at 1000C (2120F) and freeze at 00C (320F). Students may think heat and cold are different, rather than being opposite ends of a continuum. Students may think heat is a substance. Students may think heat is not energy. Performance Indicators Concepts High School Science Integrated Physics and Chemistry Unit 12 PI 01 Create a poster presentation that describes how thermal energy transfer allows a system, such as the Key Understandings For Learners Properties – Energy Energy is conserved in a closed Models – Energy human body, heat pumps, or weather, to function. Include the method of thermal energy transfer, convection, conduction, or radiation, at each transfer point in the system and a summary of how system. Thermal energy can be transferred by convection, conduction, and radiation. energy is conserved. Standard(s): I.2E , I.5D , I.5E ELPSELPS.c.4F , ELPS.c.5B , ELPS.c.5E , ELPS.c.5G Key Academic Vocabulary Supporting Conceptual Development Conduction - transfer of energy through matter by colliding particles (direct contact) Convection - transfer of heat energy by the motion of heated particles in a fluid Radiation - transfer of energy in the form of electromagnetic waves (no direct contact) 110327,110334,1 TEKS# SE# I.2 TEKS Unit Level Specificity Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2E communicate valid conclusions. Communicate VALID CONCLUSIONS Including, but not limited to: I.3 Communicate conclusions in oral, written, and graphic forms. Use essential vocabulary of the discipline to communicate conclusions. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Present scientific information in appropriate formats for various audiences. Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A in all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical Analyze, Evaluate, Critique SCIENTIFIC EXPLANATIONS reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by Including, but not limited to: Using o o o o o the student Empirical evidence Scientific evidence Logical reasoning Experimental and observational testing Critical thinking TxCCRS Note: I. Nature of Science – A1 – Utilize skepticism, logic, and professional ethics in science. I. Nature of Science – A4 – Rely on reproducible observations of empirical evidence when constructing, analyzing, and evaluating explanations of natural events and processes. I.5 Science concepts.. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to: I.5D investigate the law of conservation of energy Investigate THE LAW OF CONSERVATION OF ENERGY Including, but not limited to: Gravitational potential energy Kinetic energy Conversion between o KE = kinetic energy o o GPE = gravitational potential energy How kinetic and potential energy relate to ME(mechanical energy) Mechanical energy = kinetic energy = potential energy ME = PE + KE Calculate the kinetic energy of an object given its mass and velocity. o Kinetic energy = ½(mass)(velocity)2 o KE = 1/2mv2 Calculate the gravitational potential energy of an object given its mass and its height. o Gravitational potential energy = (mass)(acceleration due to o gravity)(height) PEg = mgh Introduce chemical potential energy. Analyze energy transformation. o Potential to kinetic TxCCRS Note: VII. Chemistry – H1 – Understand the Law of Conservation of Energy and processes of heat transfer. VIII. Physics – D2 – Understand conservation of energy. I.5E investigate and demonstrate the movement of thermal energy through solids, liquids, and gases by convection, conduction, Investigate, Demonstrate THE MOVEMENT OF THERMAL ENERGY and radiation such as in weather, living, and mechanical systems Including, but not limited to: Using kinetic theory to describe the movement of heat through o o o Solids Liquids o o o Convection Conduction o o Weather systems Living systems Gases By Radiation Evaporation, condensation, and insulation as they apply to Sweating Panting o Mechanical systems Thermal energy Heat Temperature Temperature conversion o K and °C Specific heat Heat gained or lost = (mass)(specific heat)(change in temperature) o Q = mcp∆T; Q = m X ∆T, X C TxCCRS Note: VII. Chemistry – H1 – Understand the Law of Conservation of Energy and processes of heat transfer. VII. Chemistry – I1 – Understand the behavior of matter in its various states: solid, liquid, and gas. VIII. Physics – A2 – Understand states of matter and their characteristics. VIII. Physics – H1 – Understand the gain and loss of heat energy in matter. VIII. Physics – H2 – Understand the basic laws of thermodynamics. 14 TEKS# SE# Process TEKS I.1 Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A Know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section. I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. I.3B Communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials. I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# ELPS.c.4 Subsection C: Cross-curricular second language acquisition essential knowledge and skills. Cross-curricular second language acquisition/reading. The ELL reads a variety of texts for a variety of purposes with an increasing level of comprehension in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in reading. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations apply to text read aloud for students not yet at the stage of decoding written text. The student is expected to: ELPS.c.4F use visual and contextual support and support from peers and teachers to read grade-appropriate content area text, enhance and confirm understanding, and develop vocabulary, grasp of language structures, and background knowledge needed to comprehend increasingly challenging language ELPS.c.5 Cross-curricular second language acquisition/writing. The ELL writes in a variety of forms with increasing accuracy to effectively address a specific purpose and audience in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in writing. In order for the ELL to meet grade-level learning expectations across foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations do not apply until the student has reached the stage of generating original written text using a standard writing system. The student is expected to: ELPS.c.5B write using newly acquired basic vocabulary and content-based grade-level vocabulary ELPS.c.5E Employ increasingly complex grammatical structures in content area writing commensurate with grade-level expectations, such as: (i) using correct verbs, tenses, and pronouns/antecedents; (ii) using possessive case (apostrophe s) correctly; and (iii) using negatives and contractions correctly. ELPS.c.5G narrate, describe, and explain with increasing specificity and detail to fulfill content area writing needs as more English is acquired. INSTRUCTIONAL FOCUS DOCUMENT HIGH SCHOOL COURSES SCIENCE/INTEGRATED PHYSICS AND CHEMISTRY UNIT : 13 TITLE : Unit 13: Energy: Societal Impacts SUGGESTED DURATION : 5 days Printer Friendly Version 12_S_IPC_13_IFD.pdf State Resources: Rationale This unit bundles student expectations that address the relationships between different energy resources. Prior to this unit, in elementary and middle school, students studied energy resources and types of energy. In this unit, students investigate and compare the economic and environmental impacts of using different energy resources. According to the American Association for the Advancement of Science (AAAS) in the Benchmarks for Science Literacy (Project 2061) [online version], “by the end of the 12th grade, students should know that “scientists can bring information, insights, and analytical skills to bear on matters of public concern. Acting in their areas of expertise, scientists can help people understand the likely causes of events and estimate their possible effects.” This content is reinforced while discussing the concepts of energy and energy conservation. American Association for the Advancement of Science. (1993). Benchmarks on-line. Retrieved April 25, 2008, from http://www.project2061.org/publications/bsl/online/bolintro.htm. Misconceptions MISCONCEPTION: Students may think energy resources and energy are the same. Performance Indicators Concepts Key Understandings For Learners High School Science Integrated Physics and Chemistry Unit 13 PI 01 Given a real world scenario involving an inherent energy problem, analyze economic and environmental factors to determine what energy resource would be the most effective to solve the issue. Create a Properties – Energy Energy exists in many different forms. Nature of Science Energy usage can have environmental impacts. presentation, and include graphics to persuade classmates this is the best solution to the problem. Include reasons other resources would not be as effective. Standard(s): I.2D , I.2E , I.5H , I.5I ELPSELPS.c.1C , ELPS.c.3G , ELPS.c.3H , ELPS.c.5G Key Academic Vocabulary Supporting Conceptual Development Environment – the totality of surrounding conditions Society – an extended social group having a distinctive cultural and economic organization 110327,110327,1 TEKS# SE# I.1 TEKS Unit Level Specificity Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1B demonstrate an understanding of the use and Demonstrate conservation of resources and the proper disposal or recycling of materials. AN UNDERSTANDING OF THE USE AND CONSERVATION OF RESOURCES AND THE PROPER DISPOSAL OR RECYCLING OF MATERIALS Including, but not limited to: Use and conservation of resources o Reducing pollution o o o I.2 Being a wise consumer Decreasing reliance on fossil fuels Preserving habitats Proper disposal or recycling of materials Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2D organize, analyze, evaluate, make inferences, and predict trends from data Organize, Analyze, Evaluate, Make inferences, Predict TRENDS FROM DATA Including, but not limited to: Use appropriate mathematical calculations. o Possible examples may include Averaging Percent change Probabilities and ratios Rate of change Use appropriate standard international (SI) units. Analyze data using different modes of expression (narrative, numerical, graphical). Accurately predict trends from data. TxCCRS Note: 1. I.2E communicate valid conclusions. Nature of Science – A4 – Rely on reproducible observations of empirical evidence when constructing, analyzing, and evaluating explanations of natural events and processes. Communicate VALID CONCLUSIONS Including, but not limited to: I.3 Communicate conclusions in oral, written, and graphic forms. Use essential vocabulary of the discipline to communicate conclusions. Use appropriate writing practices consistent with scientific writing. Use charts and graphs to represent data and conclusions. Present scientific information in appropriate formats for various audiences. Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3C draw inferences based on data related to promotional materials for products and services Draw INFERENCES Including, but not limited to: Examine data from promotional materials described in print, on television, and on the Internet. Evaluate data from promotional materials for quality and accuracy. Evaluation of o o o o I.5 Quality Accuracy Completeness Reliability of information from sources Science concepts.. The student recognizes multiple forms of energy and knows the impact of energy transfer and energy conservation in everyday life. The student is expected to: I.5H analyze energy conversions such as those from Analyze radiant, nuclear, and geothermal sources fossil fuels such as coal, gas, oil the movement of water or wind ENERGY CONVERSIONS Including, but not limited to: Radiant (solar) Nuclear Geothermal Fossil fuels o o o I.5I Coal Gas Oil Hydroelectric Wind critique the advantages and disadvantages of various Critique energy sources and their impact on society and the environment. THE ADVANTAGES AND DISADVANTAGES OF VARIOUS ENERGY SOURCES AND THEIR IMPACT ON SOCIETY AND THE ENVIRONMENT Including, but not limited to: Rechargeable vs. disposable batteries Solar cells Determine the amount of electric power in a system. o Energy = (power)(time) o E = Pt o Electrical power = (voltage)(current) o P = VI Explain why people are continually trying to conserve energy sources. Differentiate between batteries and generators. TxCCRS Note: VIII. Physics – I8 – Relate electricity and magnetism to everyday life. 18 TEKS# SE# I.1 Process TEKS Scientific processes.. The student, for at least 40% of instructional time, conducts laboratory and field investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: I.1A Demonstrate safe practices during laboratory and field investigations. I.1B Demonstrate an understanding of the use and conservation of resources and the proper disposal or recycling of materials. I.2 Scientific processes.. The student uses scientific methods during laboratory and field investigations. The student is expected to: I.2A Know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section. I.2B Plan and implement investigative procedures, including asking questions, formulating testable hypotheses, and selecting equipment and technology. I.2C Collect data and make measurements with precision. I.2D Organize, analyze, evaluate, make inferences, and predict trends from data. I.2E Communicate valid conclusions. I.3 Scientific processes.. The student uses critical thinking, scientific reasoning, and problem solving to make informed decisions. The student is expected to: I.3A In all fields of science, analyze, evaluate, and critique scientific explanations by using empirical evidence, logical reasoning, and experimental and observational testing, including examining all sides of scientific evidence of those scientific explanations, so as to encourage critical thinking by the student. I.3B Communicate and apply scientific information extracted from various sources such as current events, news reports, published journal articles, and marketing materials. I.3C Draw inferences based on data related to promotional materials for products and services. I.3D Evaluate the impact of research on scientific thought, society, and the environment. I.3E Describe connections between physics and chemistry and future careers. I.3F Research and describe the history of physics and chemistry and contributions of scientists. 16 The English Language Proficiency Standards (ELPS), as required by 19 Texas Administrative Code, Chapter 74, Subchapter A, §74.4, outline English language proficiency level descriptors and student expectations for English language learners (ELLs). School districts are required to implement ELPS as an integral part of each subject in the required curriculum. School districts shall provide instruction in the knowledge and skills of the foundation and enrichment curriculum in a manner that is linguistically accommodated commensurate with the student’s levels of English language proficiency to ensure that the student learns the knowledge and skills in the required curriculum. School districts shall provide content-based instruction including the cross-curricular second language acquisition essential knowledge and skills in subsection (c) of the ELPS in a manner that is linguistically accommodated to help the student acquire English language proficiency. http://ritter.tea.state.tx.us/rules/tac/chapter074/ch074a.html#74.4 ELPS# ELPS.c.1 Subsection C: Cross-curricular second language acquisition essential knowledge and skills. Cross-curricular second language acquisition/learning strategies. The ELL uses language learning strategies to develop an awareness of his or her own learning processes in all content areas. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.1C use strategic learning techniques such as concept mapping, drawing, memorizing, comparing, contrasting, and reviewing to acquire basic and grade-level vocabulary ELPS.c.3 Cross-curricular second language acquisition/speaking. The ELL speaks in a variety of modes for a variety of purposes with an awareness of different language registers (formal/informal) using vocabulary with increasing fluency and accuracy in language arts and all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in speaking. In order for the ELL to meet grade-level learning expectations across the foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. The student is expected to: ELPS.c.3G express opinions, ideas, and feelings ranging from communicating single words and short phrases to participating in extended discussions on a variety of social and grade-appropriate academic topics ELPS.c.3H narrate, describe, and explain with increasing specificity and detail as more English is acquired ELPS.c.5 Cross-curricular second language acquisition/writing. The ELL writes in a variety of forms with increasing accuracy to effectively address a specific purpose and audience in all content areas. ELLs may be at the beginning, intermediate, advanced, or advanced high stage of English language acquisition in writing. In order for the ELL to meet grade-level learning expectations across foundation and enrichment curriculum, all instruction delivered in English must be linguistically accommodated (communicated, sequenced, and scaffolded) commensurate with the student's level of English language proficiency. For Kindergarten and Grade 1, certain of these student expectations do not apply until the student has reached the stage of generating original written text using a standard writing system. The student is expected to: ELPS.c.5G narrate, describe, and explain with increasing specificity and detail to fulfill content area writing needs as more English is acquired.