Modified Oligonucleotides Containing Diphosphodiester
advertisement

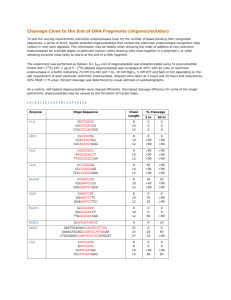
Licensing Opportunity from the University of Rhode Island Modified Oligonucleotides Containing Diphosphodiester Internucleotide Linkages Description of Invention: The synthesis and biochemical utility of modified oligonucleotides containing diphosphodiester internucleotide linkages. The synthesis of these compounds was carried out using diphosphitylating reagents. Oligonucleotides containing diphosphate diester bridges wherein said oligonucleotides are synthesized via a solid-phase synthesis strategy to form modified oligonucleotides. Diphosphitylating, triphosphitylating, tetraphosphitylating, .beta.-triphosphitylating, bifunctional diphosphitylating, bifunctional triphosphitylating, and bifunctional tetraphosphitylating reagents wherein, the phosphorus atoms are linked together through oxygen, sulfur, amino, or methylene groups and/or are substituted with chlorine, diisopropylamine and cyanoethoxy groups. Potential Areas of Application: 1) During the past two decades, chemically modified oligodeoxynucleotides (ODNs) have received much attention in search for potential therapeutic and diagnostic agents and in the study of numerous biochemical and biological processes. The use of modified ODNs as specific targeting of gene expression has become an indispensable tool in antisense research. 2) The reagents used for synthesizing these modified oligonucleotide molecules may be highly valuable in pharmaceutical and chemical industry. Main Advantages of Invention: 1) This invention describes the synthesis and potential biochemical utility of modified oligonucleotides containing diphosphodiester internucleotide linkages. The synthesis of these compounds was carried out by using novel diphosphitylating reagents. The reagents used for synthesizing these modified oligonucleotide molecules may be highly valuable in pharmaceutical and chemical industry. 2) Described is the synthesis and potential biochemical utility of modified oligonucleotides containing diphosphate diester internucleotide linkages (FIG. 1). The compounds have a larger distance between 5' and 3' oxygens of two nucleotides across the internucleotide bridge compared to that in the natural ODNs. The potential of modified ODNs for forming a double-stranded DNA by binding with modified and unmodified complementary chains was investigated and compared with natural DNA chains to establish a new family of chemically modified ODNs. Lead Inventor: Keykavous Parang et al, Biomedical Sciences Status: US patent application 11/972,254 filed January 10, 2008 Modified Oligonucleotides USPTO Website Link Category: Biological, Pharmaceutical, Life Sciences Licensing Status: Available for licensing Reference #:8011 Please contact David R. Sadowski or Raymond Walsh - Division of Research & Economic Development, University of Rhode Island, 75 Lower College Rd. Suite 001, Kingston, RI 02881; 401-874-4807 or Fax 401-874-7832 http://www.uri.edu/research/tro/executive/sadowski.html Rev. 01-14-10