Research Session: Ethical Approval
advertisement
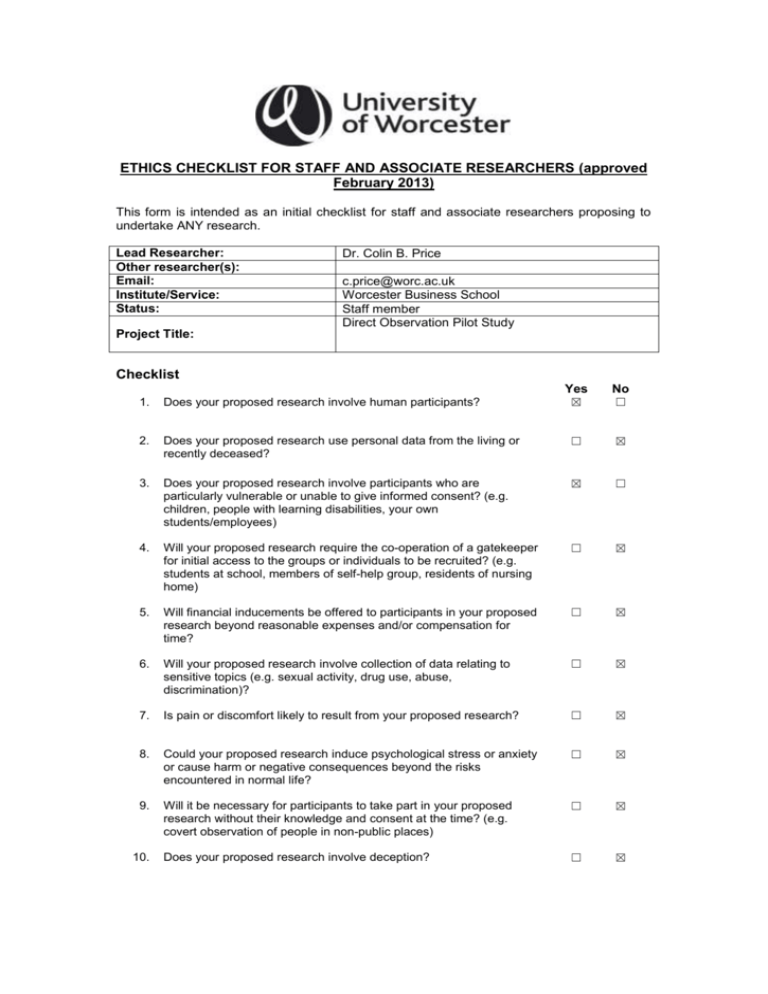
ETHICS CHECKLIST FOR STAFF AND ASSOCIATE RESEARCHERS (approved February 2013) This form is intended as an initial checklist for staff and associate researchers proposing to undertake ANY research. Lead Researcher: Other researcher(s): Email: Institute/Service: Status: Dr. Colin B. Price c.price@worc.ac.uk Worcester Business School Staff member Direct Observation Pilot Study Project Title: Checklist Yes No 1. Does your proposed research involve human participants? ☒ ☐ 2. Does your proposed research use personal data from the living or recently deceased? ☐ ☒ 3. Does your proposed research involve participants who are particularly vulnerable or unable to give informed consent? (e.g. children, people with learning disabilities, your own students/employees) ☒ ☐ 4. Will your proposed research require the co-operation of a gatekeeper for initial access to the groups or individuals to be recruited? (e.g. students at school, members of self-help group, residents of nursing home) ☐ ☒ 5. Will financial inducements be offered to participants in your proposed research beyond reasonable expenses and/or compensation for time? ☐ ☒ 6. Will your proposed research involve collection of data relating to sensitive topics (e.g. sexual activity, drug use, abuse, discrimination)? ☐ ☒ 7. Is pain or discomfort likely to result from your proposed research? ☐ ☒ 8. Could your proposed research induce psychological stress or anxiety or cause harm or negative consequences beyond the risks encountered in normal life? ☐ ☒ 9. Will it be necessary for participants to take part in your proposed research without their knowledge and consent at the time? (e.g. covert observation of people in non-public places) ☐ ☒ Does your proposed research involve deception? ☐ ☒ 10. 11. Will your proposed research require the gathering of information about unlawful activity? ☐ ☒ 12. Does your proposed research involve access to, or the collection of, sensitive/confidential data from other organisations? ☐ ☒ 13. Will invasive procedures be part of your proposed research (e.g. blood sampling, temperature probes)? ☐ ☒ 14. Will your proposed research involve prolonged, high intensity or repetitive testing? ☐ ☒ 15. Does your proposed research involve the testing or observation of animals? ☐ ☒ 16. Does your proposed research involve collection of DNA, cells, tissues or other samples from humans or animals? ☐ ☒ 17. Does your proposed research involve human remains or burial sites? ☐ ☒ 18. Does your proposed research involve NHS patients, staff or premises? ☐ ☒ 19. Is your proposed research externally funded? ☐ ☒ If the answers to any of these questions change during the course of your research, you must alert your Institute Ethics Coordinator. Signature By signing below I declare that I have answered the questions above honestly and to the best of my knowledge: CBPrice Lead researcher: Date: 30-07-14 (Please note that the Lead Researcher is, where applicable, signing on behalf of all researchers involved with the research. Electronic signatures are only valid if the signee then emails the form from their own university email address to the recipient.) What next? If you have answered NO to all of the questions, you do not need to complete the next three pages and you should now email this signed and dated checklist to your Institute Ethics Coordinator; wbsethics@worc.ac.uk. If you have answered YES to one or more questions, you must also complete the next three pages, unless you have answered yes to q.18 in which case you must complete the relevant NHS ethical review process (seek advice from the NHS authority that you intend to do your research within. If they advise that no formal NHS approval is needed, you will still need to obtain written confirmation of this). Only after pages 1-4 have been completed, you should email this signed and dated checklist to your Institute Ethics Coordinator. Application for Ethical Approval (Staff and Associate Researcher) Details of the research Outline the context and rationale for the research, the aims and objectives of the research and the methods of data collection We are interested in researching whether individuals without a scientific background can make “direct observations” on physics experiments presented in a virtual lab and describe the main features of the experimental behaviour understood by the physics experts. By “direct observations” we mean simply observing the visual representation of an experiment in action, without access to any numbers or theory. Consider the example of the experiment shown below consisting of a glider tethered by two springs. The behaviour of this experiment is as follows: (i) The glider “oscillates” from left to right, (ii) the time for each “cycle of oscillation” is the same, (iii) the maximum displacement to the left is the same as the maximum displacement to the right, (iv) these displacements are equal. We are interested to see if participants can “see” this behavior. We do not expect participants to use technical language, indeed it will be interesting to code their observations made in “plain English” onto the technical vocabulary. Methodology. This is straightforward. Participants will be shown a number of virtual experiments and will be asked one question: “Describe in as much detail as you can what you observe happening”. Their responses will be voice-recorded and subsequently analysed. The voice recording will then be destroyed on the same day as individuals’ participation. This research forms part of a larger program of study, to establish guidelines for the design of virtual physics laboratories. Who are your participants/subjects? Adults including students at the University of Worcester whom are selected to have had no experience or recent experience of Physics and Physics experimentation How do you intend to recruit your participants? This should explain the means by which participants in the research will be recruited. If any incentive and/or compensation (financial or other) is to be offered to participants, this should be clearly explained and justified. Recruitment through network of personal contacts and through students known to the researcher. No incentive will be employed and participation will be voluntary. How will you gain informed consent/assent? Where you will provide an information sheet and/or consent form, please append this. If you are undertaking a deception study or covert research please outline how you will debrief participants below Attached Confidentiality, anonymity, data storage and disposal Provide explanation of any measures to preserve confidentiality and anonymity, including specific explanation of data storage and disposal plans. No identification is requested. The voice recording will be analysed on the day of the event and immediately destroyed. No information identifying the participants will be recorded. Ethical considerations and potential risks to participants/subjects Outline the ethical issues you think the research raises and how you intend to address these issues. There is no risk to participants and no ethical issues other than involving human participants. Published ethical guidelines to be followed Identify the professional code(s) of practice and/or ethical guidelines relevant to the subject domain of the research. The form has been written in line with Saunders, M. N. K., Lewis, P. & Thornhill, A. 2012. Research methods for business students. 6th ed. Harlow, England: FT Prentice Hall Declaration of Researcher I have read the University Ethics Policy and any relevant codes of practice or guidelines and I have identified and addressed the ethical issues in my research honestly and to the best of my knowledge C.B.Price Signature*: Date: 10-08-14 What next? Email this form to your Institute Ethics Coordinator; h.watts@worc.ac.uk who will liaise with other Ethics Representatives and email you with the decision. If approved, the approval is subject to any advice which may have been given being followed. Institute Ethics Coordinator Declaration ☐ The application is approved without any changes needed ☒ The application is approved subject to revisions being made to the satisfaction of the committee ☐ The application is not approved ☐ The application is referred to the Ethics and Research Governance Committee WBS Committee comments: Needs to be made clear that as the results are anonymised, they cannot be withdrawn after the experiment has been completed. Right to withdraw needs including (a right not to participate is different). An additional, alternative email address (wbsethics@worc.ac.uk) should be included as well as Colin’s if the participants have any questions or concerns. Would advise a week turnaround for keeping interview data to allow enough time for transcription could be stated therefore all audio recordings will be destroyed once transcribed (a maximum of seven days after the experiment). Helen Watts Signature*: Date: 02/09/2014 Chair of the Ethics & Research Governance Committee Declaration ☐ The application is approved without any changes needed ☐ The application is approved subject to revisions being made to the satisfaction of the committee ☐ The application is not approved ERGC Comments: Signature*: Date: *Electronic signatures are only valid if the signee then emails the form from their own university email address to the recipient.