preliminary ethical review form
advertisement

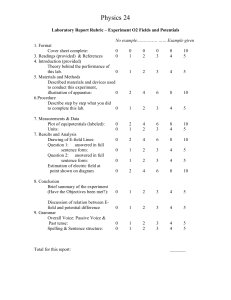
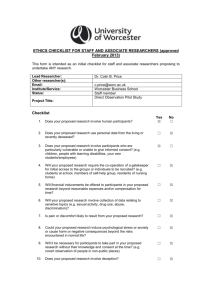
Preliminary Ethical Assessment Form As part of its assurances and compliance processes the University ensures that all appropriate projects, including student research and consultancy projects, undergo appropriate ethical review before commencement. This form is used identify high risk projects which may require further full ethical review. Additional guidance can be found at: http://www.ncl.ac.uk/res/research/ethics_governance/ethics/index.htm SECTION 1: Applicant Details Name of Researcher (Applicant): Academic Unit: Email Address: Supervisor Name (if applicable): Supervisor Email (if applicable): Module Code(if applicable): SECTION 2: Project Details Project Title: Project Synopsis: Has ethical approval to cover this proposal already been obtained? If YES, please confirm: YES NO Approving Body: Reference Number: Date of Approval: Will anyone be acting as sponsor under the NHS Research Governance Framework for Health and Social Care? Do you have a NUTH reference? YES NO If ‘Yes’ please enter the name of the sponsor: ...................... YES NO If ‘Yes’ please enter the reference: ...................... If you already have approval then you do not need to complete the rest of the form. Please go directly to the Declaration in Section 8. SECTION 3: Animals YES NO “Does your research involve the observation, capture or manipulation of animals or their tissues?” If you answered NO to this question, please go to Section 5 If you answered YES to this question, please complete the rest of the questions below. Document1 Page 1 Preliminary Ethical Assessment Form The Animals (Scientific Procedures) Act defines “protected animals” as: “any living vertebrate other than man….. in its foetal, larval or embryonic form…….from the stage of its development when— (a)in the case of a mammal, bird or reptile, half the gestation or incubation period for the relevant species has elapsed; and (b)in any other case, it becomes capable of independent feeding.” YES NO In practice 'Protected' animals are all living vertebrates (other than man), including some immature forms, and cephalopods (e.g. octopus, squid, cuttlefish). Using the definition above, does your programme of research involve you or any related party? a. Observing ‘protected animals’ within their natural or an artificial environment? Observation can be passive i.e. bird watching, active i.e. GPS tagging of individual animals, it can relate to an individual animals or collection of animals i.e. a school of fish, direct or indirect. b. Capturing ‘protected animals’ (permanently or temporarily)? c. Manipulating ‘protected animals’? Manipulation covers any experimentation within a laboratory environment, handling, care, husbandry or other interaction with animals. d. Using any data obtained by the observation, capture or manipulation of protected animals? If you answered YES to Section 3, you will need to submit an application to the Animal Welfare Ethical review Board. Please continue with the rest of the form. SECTION 4: NHS, Health & Social Care: Facilities, Staff & Patients YES NO “Will the study involve participants recruited by virtue of being service users, their dependents, their carers or human tissues or the use of NHS & Health / Social Care Facilities or otherwise require REC approval? (If you are unsure please tick ‘Yes and complete the sub-questions) If you answered NO to this question, please go to Section 5 If you answered YES to this question, please complete the rest of the questions below. Will the study involve any of the following? YES NO a. Patients and users of the NHS? b. Relatives or carers of patients and users of the NHS? c. Foetal material, Human Tissues or IVF involving NHS patients? d. The recently dead in NHS premises? e. Requires the use of, or access to NHS premises of facilities (labs, clinics) or the study is a clinical trial? Document1 Page 2 Preliminary Ethical Assessment Form f. Participants aged 16 or over who are unable to give informed consent e.g. people with learning disabilities. For a full list see the Mental Capacity Act 2005? g. Human participants (users) in a social care setting within the UK and N. Ireland? h. Intergenerational studies in social care, involving adults, children, or families as research participants? i. Or will the study come under the remit of GAFREC? If you answered YES to any of Section 4, you need to submit an application to the appropriate external health authority ethics committee through the National Research Ethics Service (NRES) – see http://www.hra.nhs.uk/hra/ for the process. Please continue with the rest of the form. SECTION 5: Human Participants in a Non-Clinical Setting Does the research involve human participants e.g. use of questionnaires, focus groups, observation, surveys or labbased studies involving human participants? (If you are unsure please tick ‘Yes’ and complete the sub-questions) YES NO If you answered NO to this question, please go to Section 6 If you answered YES to this question, please complete the rest of the questions below. Does the study involve any of the following? YES NO a. The study involves children, or other vulnerable groups; as defined in Section 59 of the Safeguarding Vulnerable Adults Act 2006 as those who are relatively or absolutely incapable of protecting their own interests, or those in unequal relationships e.g. participants who are subordinate to the researcher(s) in a context outside the research? b. The study requires the co-operation of a gatekeeper for initial access to the groups or individuals to be recruited e.g. students at school, members of a self-help group, or residents of a nursing home? c. It is necessary for participants to take part in the study without their knowledge and consent e.g. covert observation of people in non-public places? d. Deliberately misleading participants in any way? e. Discussion of sensitive topics e.g. sexual activity or drug use? f. The administration of drugs, placebos or other substances (e.g. food substances, vitamins) to the study participants g. Invasive, intrusive or potentially harmful procedures of any kind?* h. Obtaining blood or tissue samples?* Document1 Page 3 Preliminary Ethical Assessment Form i. Pain or more than mild discomfort? j. Psychological stress, anxiety, harm or negative consequences beyond that encountered in normal life? k. Prolonged or repetitive testing. i.e. more than 4 hours commitment or attendance on more than two occasions? l. Financial inducements (other than reasonable expenses and compensation for time)? * Please Note: Depending on the details of this project, this may require NHS approval. You will be given further clarification if the project is awarded. You are also advised to consult the JRO Policy Regarding the Participation of Volunteers in Research Projects. If you have answered YES to any of questions in Section 5: You need to submit an application to the appropriate Faculty Ethics Committee before your project progresses. Please continue with the rest of the form. SECTION 6: Data YES NO Does the research involve the usage or transfer of Sensitive personal data as defined as by the Data Protection Act 1998 or data governed by statute such as the Official Secrets Act, commercial contract or by convention e.g. client confidentiality? (If you are unsure please tick ‘Yes’ and complete the sub-questions) If you answered NO to this question, please go to Section 7 If you answered YES to this question, please complete the rest of the questions below. YES NO a. Will the study involve the sharing of sensitive data outside the European Economic Area? b. Will the study involve the collection or analysis of sensitive data which will be identifiable within the project outputs and could potentially cause harm? c. Will the study involve the collection or analysis of personal data without explicit consent? d. Will the study involve the collection or analysis of information covered by the Official Secrets Act, Terrorism Act, commercial contract or license? If you have answered YES to any of questions in Section 6: You need to submit an application to the appropriate Faculty Ethics Committee before your project progresses. Please continue with the rest of the form. SECTION 7: Environment Will the study cause direct or indirect damage to the environment or emissions outside permissible levels or be conducted in an area of special scientific or cultural interest? (If you are unsure please tick ‘Yes’ and complete the sub-questions) If you answered NO to this question, please go to section 8 Document1 YES NO Page 4 Preliminary Ethical Assessment Form If you answered YES to this question, please complete the rest of the questions below. YES NO a. Is the research expected to lead to emissions to land, air or water above the permissible level according to UK regulations (or local regulations in the case of non-UK research)? b. Is the research expected to lead to a detrimental effect to the landscape or cultural heritage, including artefacts? c. Is it expected that the research might cause harm through environmental fieldwork such as sampling or monitoring a site? d. Will the research be conducted in an environmentally sensitive area or area of special scientific interest? If you have answered YES to any of questions in Section 7: You need to submit an application to the appropriate Faculty Ethics Committee before your project progresses. Please continue with the rest of the form. SECTION 8: International Projects YES NO Will the research be conducted outside of the European Economic Area (EEA) or will it involve international collaborators outside the EEA? If you have answered YES to any of questions in Section 8: You need to submit an application to the appropriate Faculty Ethics Committee before your project progresses. Please continue with the rest of the form. SECTION 9: Declaration By signing this declaration you confirm that; * I certify that the information contained in this application is accurate. * I certify that the research will be undertaken in line with all appropriate local standards and regulations. * I certify that I have discussed this form with my project supervisor and that they agree with the answers given Name of Principal Investigator: Signed: Date: If you have any queries about this or any other ethical issue, please contact your Faculty Ethics Coordinator or appropriate Grants and Contracts team. -------------------------------------------------------------------------------------------------------------------------For office use only: Date received in G&C: Document1 Requires full approval: YES/NO Page 5