Step 1 - dimacleans
advertisement
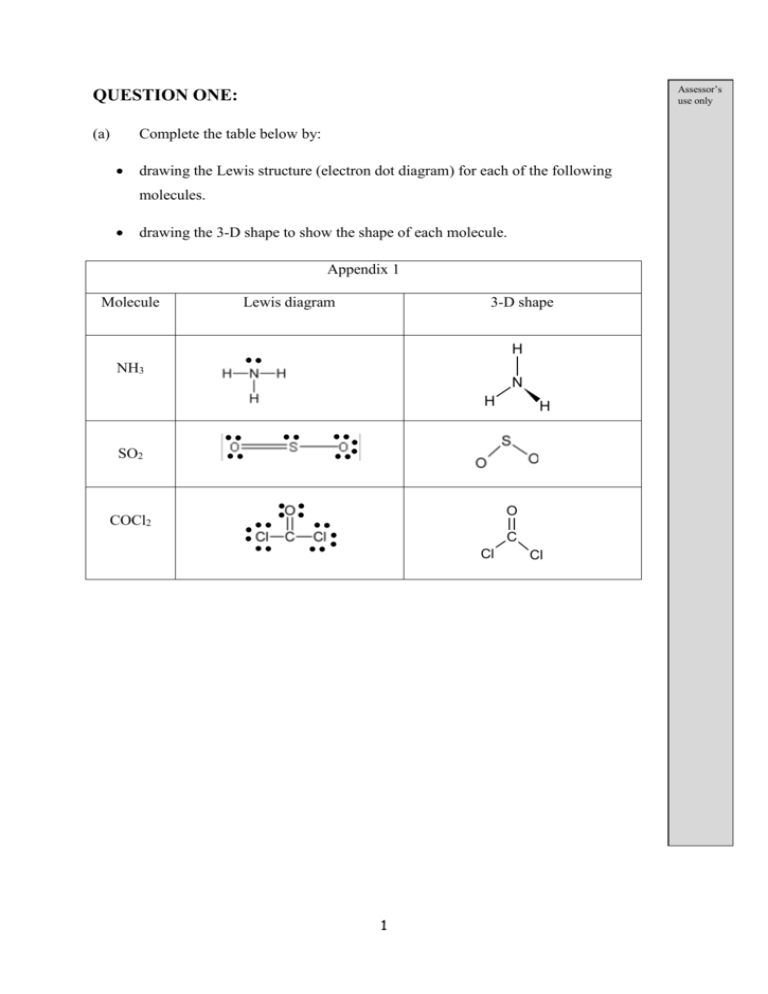
Assessor’s use only QUESTION ONE: (a) Complete the table below by: drawing the Lewis structure (electron dot diagram) for each of the following molecules. drawing the 3-D shape to show the shape of each molecule. Appendix 1 Molecule Lewis diagram 3-D shape H NH3 N H H SO2 O COCl2 C Cl 1 Cl (b) The following table shows the Lewis diagrams for the molecules H2S and SO3. Molecule H2 S Lewis S diagram H SO3 O H S O O Discuss the shapes and bond angles of these two molecules. In your answer you should include: the shape of hydrogen sulfide, H2S and sulfur trioxide, SO3. the factors that determine the shape of each molecule. the bond angle of H2S and SO3. an explanation of the bond angles of H2S and SO3. Step 1 – state the shape and bond angle: H2S is a bent (or angular) shape with a 109o bond angle Step 2 – outline the numbers of electron pairs/clouds around the central atom and the subsequent repulsion There are a total of 4 electron clouds around the central sulfur atom. These clouds are repelling each other, pointing to the 4 corners of a tetrahedron. As a consequence the angle between these pairs, and hence the bond angle is 109.5o. Step 3 – use numbers of bonding vs non-bonding electron clouds to justify the shape There are 2 bonding clouds vs 2 non-bonding clouds of electrons. The non-bonding clouds influence the shape, but are not part of the shape. Thus the molecule is bent in shape. SO3 has a trigonal planar shape with a 120o bond angle. There is a total of 3 clouds around the central sulfur atom. The clouds a repelling each other, pointing to the 3 corners of a triangle. As a consequence the angle between electron clouds is 120o. All three clouds are bonding clouds, so all three are part of the shape. Thus the molecule has a trigonal planar shape. 2 (c) The three dimensional shapes of two molecules are shown below. F F C F F N F F F Circle the word that describes the polarity of each of the molecules CF4 and NF3. CF4 polar non-polar NF3 polar non-polar For each molecule, justify your choice. Step 1 – outline the polarity of the C-F bond, with reasoning related to electronegativity differences The C-F bond is polar/a dipole, as fluorine is more electronegative than carbon. Step 2 – comment on the symmetry of the molecule and whether these dipoles cancel out The molecule is tetrahedral in shape, with the polar C-F bonds (the dipoles) symmetrically (evenly) distributed around the central carbon. As a result these dipoles cancel out. Step 3 – restate the polarity of the molecule as a consequence of what has been written Thus the CF4 molecules in non-polar The N-F bond is polar as fluorine has a greater electronegativity that nitrogen. However, the (trigonal pyramidal) is not very symmetrical OR the N-F bonds are not symmetrically (evenly) distributed around the nitrogen. As a result the dipoles of the N-F bonds do not cancel out. Thus the molecule is polar. 3 QUESTION TWO: (a) Complete the table below by stating the type of particle and the bonding (attractive force) between the particles for each of the substances. (b) Substance Type of particle Bonding between particles Copper, Cu Atoms Metallic Copper sulfate, CuSO4 Ions Ionic Contrast both the malleability and solubility in water for both copper, Cu, and copper sulfate, CuSO4, using your knowledge of structure and bonding. Step 1 – Outline the structure and bonding of a substance Copper consists of a 3D lattice of metal atoms, from which the valance electrons have become delocalised. The positive nuclei of the atoms are attracted the delocalised electrons, forming the metallic bond. Step 2 – Relate this structure to one of the properties Copper is malleable, so when the metal bends the metallic bonds are not broken. This is because the attractions involved mobile electrons. Thus as the atoms move, the electrons can also move, preventing the attractions from breaking. OR AS the electrons are delocalised, they are considered to be nondirectional, so don’t break as the metal is deformed. Step 3 – Relate the structure to the second property The metallic bond is very strong. For a substance to dissolve the attractions between its particles and water molecules needs to stronger than between its own particles. Attractions between water molecules and copper atom are relatively weak, so copper does not dissolve. 4 Copper sulfate consists of a 3D lattice of ions. The oppositely charged ions (Cu2+ and SO42-) are attracted to one another. The attraction between the ions is called an ionic bond. As the ions are in a rigid lattice (i.e. no particles are free to move), the ionic bond is directional. Hence, when CuSO4 is deformed, ions of the same charge are forced next to each other, leading to repulsion, and the ionic bonds break. So CuSO4 is not malleable. Water molecules are polar, and can form strong attractions to ions. When CuSO4 is mixed with water, the slightly positive Hδ+ attracts the SO42- and the slightly negative Oδ- attracts the Cu2+. So the ions are attracted out of the lattice and CuSO4 is soluble. (c) Carbon dioxide, CO2, and silicon dioxide, SiO2, are both group 14 oxides. (i) Discuss the difference in structure and bonding between carbon dioxide and silicon dioxide. Describe the structure and bonding in each of the substances given CO2 consists of molecules joined by weak intermolecular forces. An individual molecule consists of one C atom bonded covalently to two oxygen atoms. SiO2 consists of silicon and oxygen atoms connected with covalent bonds termed a covalent network (3D lattice). (ii) Use your answer above to justify the difference in melting point between carbon dioxide, CO2 and silicon dioxide, SiO2. Step 1 – state the melting point of each substance CO2 has a low melting point; it is a gas at room temperature. Whereas SiO2 has high melting point, making it a solid at room temperature. Step 2 – use the structure outlined above to justify these properties When CO2 is melted, the intermolecular (van der Waals) forces are broken. These forces are weak, only a small amount of heat energy is needed to break the forces. So CO2’s melting point is low. When SiO2 is melted, the covalent bonds need to be broken. As these bonds are strong, a large amount of heat energy is needed to break the bonds. So SiO2’s melting point is high. 5 QUESTION THREE: Below is a drawing of two water molecules. Each individual molecule is drawn inside a box for clarity. O H (a) H H O H With reference to the diagram above, describe the bonds that are broken when water boils. Answer by referring to a feature of the diagram The dotted lines in the diagram are broken (b) In a well-known laboratory reaction, water vapour can be formed by burning hydrogen in oxygen gas (air). This is shown in the equation 1 below. Equation 1: 2H2 (g) + O2 (g) 2H2O (g) (i) State which bonds are broken and which are formed during this reaction. Bonds broken: H-H bonds and O=O (from the H2 and O2 molecules) Bonds formed: O-H bonds (in the H2O molecules) 6 (ii) Calculate the change in enthalpy for equation 1. You will need to use the values in the table below. (The values are true only for molecules in the equation above.) molecule Energy required to break 1 mol of bonds inside molecule/ kJ hydrogen gas 436 oxygen gas 498 water as a gas 464 Step 1 – calculate the energy required to break the bonds in the reactants; care when counting the number of bonds Bonds broken: 2 x H-H = 2 x 436 = 872 1 x O=O = 1 x 498 = 498 1370 Step 2 – calculate the total energy of the bonds formed in the product – you need to know the structure of the product here i.e. H-O-H, so 2 O-H bonds per molecule, and there are 2 of them Bonds formed: 4 x O-H = 4 x 464 = 1856 Step 3 – calculate the change in enthalpy using: Enthalpy change = bond broken – bond formed = 1370 – 1856 = -486 kJ mol-1 7 (c) Another common reaction producing water is that of the combustion of methane. This is represented in equation 2 below. Equation 2: CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) ΔrH = -889 kJ mol-1 (i) The Ea for this reaction is 120 kJ mol-1 under particular conditions. Sketch the reaction profile diagram in the box below for equation 2. Your diagram need not be to scale. Your diagram should have the following parts labelled (with their values where relevant): Reactants Products Enthalpy change Activation energy Reactants CH4 + 2O2 Enthalpy change = 889 kJ mol-1 Activation energy = 120 kJ mol1 Products CO2 + 2H2O 8 (d) Calculate the energy released if 10.0 g of water is produced. M (H2O) = 18.0 g mol-1 Step 1 – calculate number of moles of water formed (n(H2O)) n(H2O) = mass/molar mass = 10g/18.0 gmol-1 = 0.556 mol Step 2 – calculate the energy released when 1 mol of water is formed From equation, 890 kJ for 2 mol of H2O 890/2 = 445 kJ for 1 mol of H2O Step 3 – calculate energy released for no. of moles of H2O from step 1 ΔH = 0.556 x 445 = 247 kJmol-1 Its exothermic, so ΔH = -247 kJmol-1 9