TA_problems
advertisement

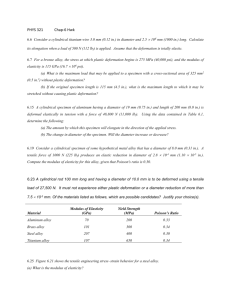
Problem 1 The net bonding energy EN between two isolated positive and negative ions is a function of interionic distance r as follows: EN = - A B + r rn (1) where A, B, and n are constants for the particular ion pair. This equation is also valid for the bonding energy between adjacent ions in solid materials. The modulus of elasticity E is proportional to the slope of the interionic force–separation curve at the equilibrium interionic separation; that is, æ dF ö E µç ÷ è dr ø r (2) o Derive an expression for the dependence of the modulus of elasticity on these A, B, and n parameters (for the twoion system), using the following procedure: Solution This problem asks that we derive an expression for the dependence of the modulus of elasticity, E, on the parameters A, B, and n in Equation (1). It is first necessary to take dEN/dr in order to obtain an expression for the force F; this is accomplished as follows: æ Aö æ Bö dç- ÷ dç ÷ dE N è rø è rn ø F= = + dr dr dr = A r (1 + 1) - nB r (n + 1) The second step is to set this dE /dr expression equal to zero and then solve for r (= r0). The algebra for this N procedure yields æ Aö r0 = ç ÷ è nB ø 1/(1 - n) Next it becomes necessary to take the derivative of the force (dF/dr), which is accomplished as follows: æ Aö æ nB ö dç ÷ dç2 èr ø è r n + 1 ÷ø dF = + dr dr dr - 2A r 3 + (n)(n + 1)B r (n + 2) Now, substitution of the above expression for r0 into this equation yields æ dF ö 2A (n)(n + 1)B + çè dr ÷ø = 3/(1 - n) (n + 2)/(1 - n) æ Aö æ Aö r0 çè nB ÷ø çè nB ÷ø which is the expression to which the modulus of elasticity is proportional. Problem 2 Compute the radius r of an impurity atom that will just fit into a BCC tetrahedral site in terms of the atomic radius R of the host atom (without introducing lattice strains). Solution A (100) face of a BCC unit cell is shown below. The interstitial atom that just fits into this interstitial site is shown by the small circle. It is situated in the plane of this (100) face, midway between the two vertical unit cell edges, and one quarter of the distance between the bottom and top cell edges. From the right triangle` defined by the three arrows we may write 2 2 æ aö æ aö 2 çè 2 ÷ø + çè 4 ÷ø = (R + r ) However, from Equation 3.4, a = 4R , and, therefore, making this substitution, the above equation takes the form 3 2 2 æ 4R ö æ 4R ö 2 2 çè ÷ø + çè ÷ = R + 2R r + r 2 3 4 3ø After rearrangement the following quadratic equation results in: r 2 + 2Rr - 0.667R 2 = 0 And upon solving for r: r= -(2R) ± (2R)2 - (4)(1)(-0.667R2 ) -2R ± 2.582R = 2 2 And, finally -2R + 2.582R = 0.291R 2 -2R - 2.582R r(-) = = - 2.291R 2 r(+) = Of course, only the r(+) root is possible, and, therefore, r = 0.291R. Problem 3 Calculate the unit cell edge length for an 80 wt% Ag 20 wt% Pd alloy. All of the palladium is in solid solution, the crystal structure for this alloy is FCC, and the room-temperature density of Pd is 12.02 g/cm3. The average density and average atomic weight for the alloy are related as follows rave = nAave VC N A Since the unit cell is cubic, then VC = a3, then rave = nAave a3 N A Solve for a æ nAave ö a=ç ÷ è rave N A ø 1/3 Expressions for Aave and ave, when incorporated into the above formula yields 1/3 é æ ö ù ê ç ÷ ú ê ç 100 ÷ ú ê nç C ÷ ú ê ç Ag + CPd ÷ ú ê çè A APd ÷ø ú Ag ú a= ê ö êæ ú êç ú ÷ êç 100 ÷N ú êç C ÷ Aú ê ç Ag + CPd ÷ ú ê çè rAg r Pd ÷ø ú ë û n=4 for FCC. The atomic weights for Ag and Pd are 107.87 and 106.4 g/mol, respectively, and the densities for the Ag and Pd are 10.49 g/cm3 and 12.02 g/cm3. Substitution gives é æ ö ê ç ÷ 100 ê (4 atoms/unit cell) ç ÷ ê ç 80 wt% + 20 wt% ÷ ê çè 107.87 g/mol 106.4 g/mol ÷ø a= ê êæ ö êç ÷ 100 êç ÷ 6.022 ´ 1023 atoms/mol ê ç 80 wt% 20 wt% ÷ + êç ÷ êë è 10.49 g/cm3 12.02 g/cm 3 ø ( = 4.050 ´ 10-8 cm = 0.4050 nm 1/3 ) ù ú ú ú ú ú ú ú ú ú ú úû