TD3 Statistical Physics (M1)
advertisement

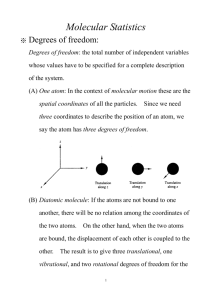
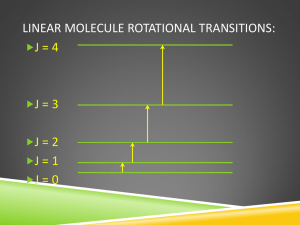
TD3 Statistical Physics (M1) Diatomic Molecules We will consider in the following a perfect gas of N identical diatomic molecules, at the temperature T in the volume V. We will first study the case of a single isolated diatomic molecule composed by two atoms with different masses mA and mB. A simple model of heteronuclear diatomic molecule consists in writing the total Hamiltonian of the molecule as the sum of 5 contributions H=Htrans+Hvib+Hrot+Hélec+Hnucl where Htrans describes the motion of the center of mass of the molecule Hvib is due to the vibration of the molecule around its equilibrium bond length Hrot characterizes the rotational motion of the molecule around its center of mass Hélec describes the electronic motion in the field created by the nuclei Hnucl describes the motion of the nucleons inside nuclei 1) Motion of the center of Mass: Write the classical Hamiltonian for the classical motion of the center of mass of the isolated molecule. Calculate Z classical the partition function corresponding to this classical motion. trans 2) Classical Vibrational Motion. The Hamiltonian of a 1D classical harmonic oscillator is: H classical vib p2 1 Kx 2 2 2 where is the “reduced mass” mAmB mA mB Calculate Z classical the partition function of this classical oscillator. vib 3) Quantum Vibrational Motion. If the vibration energy corresponds to those of a 1D quantum harmonic oscillator with the frequency w, the vibration energies are vib (n 1 / 2) . Show that the corresponding partition function can be written: Z vib exp 2k B T 1 exp k BT Give the temperature Tvib above which the vibrational behavior of the diatomic molecule is classical. 4) Classical Rotational Motion. The classical kinetic energy of the rotational part can be written in spherical coordinates as: H classical rot 1 2 2 r sin 2 .2 2 where r rA rB is the distance between the 2 particles (rA is the distance of particle A to the center of mass, rB the distance of particle B to the center of mass) and mA .mB /( mA mB ) is the effective mass of the molecule. Prove that r 2 m A rA2 mB rB2 . I r 2 is the momentum of inertia of the molecule around its center of mass. Give the expression of the effective momenta p and p . Calculate the corresponding partition function Z classical by a proper integration in phase-space. rot 5) Quantum Rotational Motion. In quantum mechanics, the rotational energy levels are given by: 2 J ( J 1) 2I Where J (0 J ) is the rotational quantum number giving the discrete values of the angular rot momentum L J ( J 1) and I is the momentum of inertia of the molecule around its center of mass. We remember that, for a given J , there are 2J 1 accessible quantum states referenced by the quantum number m( J m J ) associated to the eigenvalues of the L z operator. T 2 and x rot . 2.I.k B T Write the partition function Z rot characterizing the rotational motion of the molecule. We note Trot Give the temperature above which the vibrational behavior of the diatomic molecule is classical. In the high temperature regime (x<<1), write the contribution of the rotational motion to the free energy per molecule f rot , to the internal energy u rot , and to the Heat Capacity c V , rot . Idem in the low temperature regime (x>>1). 6) Electrons and nucleons For the last two contributions to the total partition function, Z élec and Z nucl , we suppose that only the fundamental electronic and nuclear energy levels contribute. We note elec the rotational quantum number of electrons, Selec the electronic spin, SA the nuclear spin of molecule A and SB the nuclear spin of molecule B, A and B the nuclear energy levels. Write Z élec and Z nucl . Assembly of molecules. 7) What would be the total partition function for N indistinguishable and “non-interating” diatomic heteronuclear molecules (dilute limit) in the classical limit? 8) Compute the corresponding free energy F, the internal energy U, the entropy S and the heat capacity at constant volume Cv in the classical limit. Appendix Euler – Mac Laurin Formula : f (n ) n 0 0 f (u )du 1 1 1 ( 3) f 0 f ' 0 f 0 ... 2 12 720