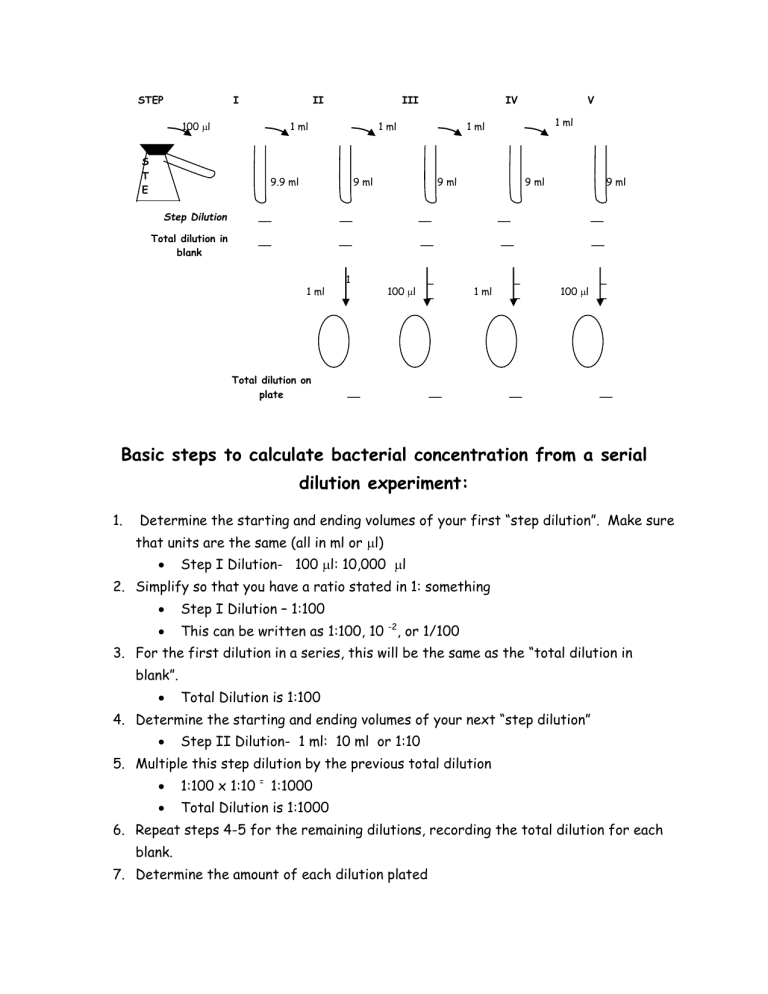
Basic steps to calculate bacterial concentration from a serial dilution
STEP I II III IV V
100 l 1 ml
S
T
E
P
9.9 ml
Step Dilution __
I
I
Total dilution in blank
I
I
I
I
I
V
V
__
1 ml
Total dilution on plate
__
__
1 m l
9 ml
__
1 ml
100 l
__
__
_
_
9 ml
__
1 ml
1 ml
__
__
_
_
__
9 ml
1 ml
100 l
__
__
_
_
9 ml
__
Basic steps to calculate bacterial concentration from a serial dilution experiment:
1.
Determine the starting and ending volumes of your first “step dilution”. Make sure that units are the same (all in ml or l)
Step I Dilution- 100 l: 10,000 l
2.
Simplify so that you have a ratio stated in 1: something
Step I Dilution – 1:100
This can be written as 1:100, 10 -2 , or 1/100
3.
For the first dilution in a series, this will be the same as the “total dilution in blank”.
Total Dilution is 1:100
4.
Determine the starting and ending volumes of your next “step dilution”
Step II Dilution- 1 ml: 10 ml or 1:10
5.
Multiple this step dilution by the previous total dilution
1:100 x 1:10 = 1:1000
Total Dilution is 1:1000
6.
Repeat steps 4-5 for the remaining dilutions, recording the total dilution for each blank.
7.
Determine the amount of each dilution plated
a) IF the amount plated is 1 ml, then the dilution represented on the plate is the SAME as the dilution blank above.
Example: 1 ml of 1:1000 would give you a plate dilution 1:1000 b) IF the amount plated is 100 l, then you must factor in an addition
1:10 dilution by multiplying by the dilution from the above blank
Example: 100 l of a 1:1000 would give you a plate dilution of
1:10,000
8.
To calculate concentration, count the colony forming units (CFUs) and multiple by the inverse of the dilution, which is called the dilution factor
Example: 79 CFUs on a 1:1000 plate would give you a concentration of 79 x
1000 CFUs/ml (or simplified to 79 x 10 3 OR 7.9 x 10 4 CFUs/ml)