Post-market surveillance of in vitro diagnostics
advertisement

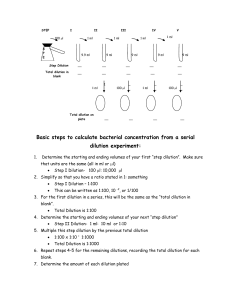
ANNEX 2 – TESTING REPORT FORMAT FOR LOT VERIFICATION TESTING 1. General information about the lot testing event Date tests received dd/mm/yyyy Test date dd/mm/yyyy Product name [add] Distributor/imp orter name and address [add] Product code [add product code] Expiry date dd/mm/yyyy Lot number [add] Pre-distribution lot testing [tick one] Post-distribution lot testing [tick one] Laboratory performing the testing [name of testing laboratory] Site test kits were sampled from [add site name] Report number [add report number assigned by testing laboratory] Report date dd/mm/yyyy Responsible person [add name] 2. Introduction The objective of lot testing is to verify the performance of the IVD and to ensure that it continues to meet WHO requirements for prequalification54 by identifying any form of product failure. 3. Materials and method The lot testing verification panel of well-characterized specimens constituted of the following specimens. The specimens were characterized with [state test kit name and manufacturer name]. Testing objective Specimen details Total Anti-HIV-1 analytical sensitivity 4 HIV-1 specimens 2-fold dilutions (first non-reactive and last two reactive specimens) 12x3 Anti-HIV-2 analytical sensitivity 1 HIV-2 specimen 2-fold dilutions (first non-reactive and last two reactive specimens) 3x 3 Sensitivity 2 (undiluted) HIV low seropositive specimens 2 Specificity 3 seronegative specimens 3 Grand total 50 The IVD was performed exactly according to manufacturer’s instructions for use. For rapid diagnostic tests and other subjectively read assays, the band intensity was scored. 54 Or continues to meet the initial performance criteria for pre-market assessment for non-WHO-prequalified IVDs. 1 POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS 4. Results and analysis The evaluation results were interpreted by two technicians, recorded in a standardized data collection worksheet. These data were then transcribed into this lot testing report. Test results Panel Specimen ID Reading 1 Series 1, Dilution 1 Series 1, Dilution 1 Series 1, Dilution 1 Series 1, Dilution 2 Series 1, Dilution 2 Series 1, Dilution 2 Series 1, Dilution 3 Series 1, Dilution 3 Series 1, Dilution 3 Series 2, Dilution 1 Series 2, Dilution 1 Series 2, Dilution 1 Series 2, Dilution 2 Series 2, Dilution 2 Series 2, Dilution 2 Series 2, Dilution 3 Series 2, Dilution 3 Series 2, Dilution 3 Series 3, Dilution 1 Series 3, Dilution 1 Series 3, Dilution 1 Series 3, Dilution 2 Series 3, Dilution 2 2 POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS Reading 2 Lot testing result Reference result POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS Test results Panel Specimen ID Reading 1 Series 3, Dilution 2 Series 3, Dilution 3 Series 3, Dilution 3 Series 3, Dilution 3 Series 4, Dilution 1 Series 4, Dilution 1 Series 4, Dilution 1 Series 4, Dilution 2 Series 4, Dilution 2 Series 4, Dilution 2 Series 4, Dilution 3 Series 4, Dilution 3 Series 4, Dilution 3 Series 5, Dilution 1 Series 5, Dilution 1 Series 5, Dilution 1 Series 5, Dilution 2 Series 5, Dilution 2 Series 5, Dilution 2 Series 5, Dilution 3 Series 5, Dilution 3 Series 5, Dilution 3 HIV + HIV + HIV HIV HIV - 3 POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS Reading 2 Lot testing result Reference result POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS 5. Acceptance criteria Specimen type Acceptance criteria for RDTs 4x HIV-1 dilution series All triplicates to be concordant for each specimen. First non-reactive specimen to be non-reactive, last reactive specimen to be reactive. 1x HIV-2 dilution series All triplicates to be concordant for each specimen. First non-reactive specimen to be non-reactive, last reactive specimen to be reactive. 2x HIV seropositive specimens Both specimens to be reactive. 3x HIV seronegative specimens All three specimens to be non-reactive. Lot testing specimens Lot number [add lot number] HIV-1 dilution series #1 [Pass/Fail] HIV-1 dilution series #2 [Pass/Fail] HIV-1 dilution series #3 [Pass/Fail] HIV-1 dilution series #4 [Pass/Fail] HIV-2 dilution series [Pass/Fail] Undiluted HIV seropositives [Pass/Fail] HIV seronegatives [Pass/Fail] 6. Conclusion Lot number [add] of [add assay name] with expiry date [add] was tested on [dd/mm/yyyy]. The results showed that this lot produced acceptable results for the lot verification testing panel. Any unexpected outcomes, e. g. instability of reagent, defects, software errors etc. as well as any deviation from the defined procedures shall be properly recorded and be part of the evaluation report. 4 POST-MARKET SURVEILLANCE OF IN VITRO DIAGNOSTICS