pola27689-sup-0001-suppinfo
advertisement

Supporting Information for
Facile Synthesis of Fluorene-based π-Conjugated Polymers via Sequential Bromination/Direct
Arylation Polycondensation
Hitoshi Saito, Junpei Kuwabara, and Takaki Kanbara
Tsukuba Research Center for Interdisciplinary Materials Science (TIMS), Graduate School of Pure and
Applied Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8573, Japan.
E-mail: kanbara@ims.tsukuba.ac.jp
Materials
Anhydrous dichloromethane and toluene were purchased from Kanto Chemical and used as a dry
solvent. 9,9-Dioctylfluorene, 2,7-dibromo-9,9-dioctylfluorene, 2,2´,3,3´,5,5´,6,6´-octafluorobiphenyl,
and other chemicals were received from commercial suppliers and used without further purification.
ZnCl2 was dried for 6 h at 150 °C under vacuum before use. Benzyltrimethylammonium tribromide
(BTMA Br3) was purchased from TCI and purified by recrystalization with dichloromethane-diethylether
(5 : 1) according to the literature.S1 5-Octylthieno[3,4-c]pyrrole-4,6-dione (TPD) was synthesized
according to literaturesS2 and purified by recrystallization from hexane.
General methods
1
H, 13C{1H} and 19F NMR spectra were recorded on Bruker AVANCE-400 and AVANCE-600 NMR
spectrometers. 1H and 13C{1H} NMR spectra were measured with tetramethylsilane (TMS) as an internal
standard. 19F NMR spectra were measured with hexafluorobenzene as an external standard (-162.9
ppm). Gel permeation chromatography (GPC) measurements were carried out on a SHIMADZU
prominence GPC system equipped with polystyrene gel columns, using CHCl3 as an eluent after
calibration with polystyrene standards. The absorption spectra were recorded on a JASCO V-630
spectrometer. MALDI-TOF-MS spectra were recorded on AB SCIEX MALDI TOF/TOF 5800 using trans-2[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB) as matrix. Elemental analyses
were carried out with a Perkin-Elmer 2400 CHN Elemental Analyzer.
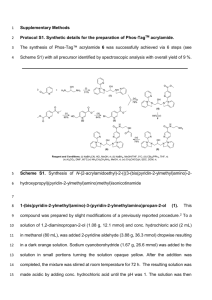
Bromination of 9,9-dioctylfluorene with benzyltrimethylammonium tribromide in acetic acid
A mixture of ZnCl2 (170 mg, 1.25 mmol), 9,9-dioctylfluorene (195 mg, 0.500 mmol), dichloromethane (1
mL), acetic acid (3 mL), and BTMA Br3 (390 mg, 1.00 mmol) was stirred for 24 h at 40 °C under nitrogen
atmosphere. After the mixture was cooled to room temperature, water (3 mL) and 5 wt% aq. solution of
NaHSO4 (2 mL) were added. The organic phase was extracted with hexane (10 mL × 3). The organic
layer was dried over magnesium sulfate and passed through a short alumina-column with
dichloromethane. The eluent was concentrated in vacuo, giving 2,7-dibromo-9,9-dioctylfluorene as a
1
white solid (269 mg, 98%). 1H NMR (400 MHz, CDCl3): δ 7.43-7.53 (m, 6H), 1.91 (m, 4H), 1.27-0.95 (m,
20H), 0.83 (t, J = 7.0 Hz, 6H), 0.58 (br, 4H).[S3]
Bromination of 9,9-dioctylfluorene with benzyltrimethylammonium tribromide in dichloromethane
A mixture of dried ZnCl2 (170 mg, 1.25 mmol), 9,9-dioctylfluorene (195 mg, 0.500 mmol), anhydrous
dichloromethane (2 mL), and BTMA Br3 (390 mg, 1.00 mmol) was stirred for 24 h at 40 °C under nitrogen
atmosphere. After the mixture was cooled to room temperature, water (3 mL) and 5 wt% aq. solution of
NaHSO4 (2 mL) were added. The organic phase was extracted with hexane (10 mL × 3). The organic layer
was dried over magnesium sulfate and passed through a short alumina-column with dichloromethane.
The eluent was concentrated in vacuo, giving 2,7-dibromo-9,9-dioctylfluorene as a white solid (267 mg,
98%).
Scheme S1. Bromination of 9,9-dioctylfluorene with BTMA Br3.
Bromination of 9-ethylcarbazole with benzyltrimethylammonium tribromide in dichloromethane
A mixture of dried ZnCl2 (170 mg, 1.25 mmol), 9-ethylcabazole (97.6 mg, 0.500 mmol), anhydrous
dichloromethane (2 mL), and BTMA Br3 (390 mg, 1.00 mmol) was stirred for 24 h at 40 °C under nitrogen
atmosphere. After the mixture was cooled to room temperature, water (8 mL) and 5 wt% aq. solution of
NaHSO4 (2 mL) were added. The organic phase was extracted with dichloromethane (10 mL × 3). The
organic layer was dried over magnesium sulfate and purified by flash chromatography
(hexane/dichloromethane 3:1). The eluent was concentrated in vacuo, giving 3,6-dibromo-9ethylcarbazole as a pale yellow solid (177 mg, 100%). 1H NMR (400 MHz, CDCl3): δ 8.15(d, J = 2.0 Hz,
2H), 7.56 (dd, J = 2.0, 8.8 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 1.41 (t, J = 7.2 Hz, 3H).
The 1H NMR data agree with those in previous report.[S3]
2
Scheme S2. Bromination of 9-ethylcarbazole with BTMA Br3.
Direct arylation polycondensation in the presence of byproducts of a bromination reaction
A catalyst solution was prepared in advance using the following procedure: a mixture of PCy3 (239 mg,
0.852 mmol), Pd(OAc)2 (95.7 mg, 0.426 mmol), and anhydrous toluene (4.25 mL) was stirred at room
temperature overnight under nitrogen atmosphere. The resulting mixture was used as a catalyst
solution (PCy3: 0.200 mol/L, Pd(OAc)2: 0.100 mol/L).
A mixture of 2,7-dibromo-9,9-dioctylfluorene (274 mg, 0.500 mmol), Cs2CO3 (652 mg, 2.00 mmol),
additives: succinimide (99.1 mg, 1.00 mmol) or Benzyltrimethylammonium bromide (BTMA Br) (230 mg,
1.00 mmol) and/or dried ZnCl2 (170 mg, 1.25 mmol) and anhydrous toluene (1.6 mL) was stirred for 1 h
under nitrogen atmosphere. Then, TPD (133 mg, 0.500 mmol), catalyst solution (0.400 mL including
Pd(OAc)2 (0.0400 mmol) and PCy3 (0.0800 mmol)), pivalic acid (PivOH) (0.0169 mL, 0.150 mmol), and
anhydrous toluene (0.5 mL) were added to the mixture. The resulting mixture was stirred at 100 °C
during indicated time. Purification was carried out according the literature.S4
Sequential bromination of 9,9-dioctylfluorene and Pd-catalyzed direct arylation polycondensation
with 2,2´,3,3´,5,5´,6,6´-octafluorobiphenyl
Poly[(9,9-dioctylfluorene-2,7-diyl)-(2,2´,3,3´,5,5´,6,6´-octafluoro-4,4’-diphenylene)] (Polymer 2) was
synthesized from 9,9-dioctylfluorene and 2,2´,3,3´,5,5´,6,6´-octafluorobiphenyl using a procedure similar
to that for Polymer 1. Polymer 2 was obtained as a pale gray solid in 81% yield (Mn = 25200, Mw/Mn =
2.38). 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 8.4 Hz, 2H), 7.59 (br, 4H), 2.07 (br, 4H), 1.01-1.33 (br,
20H), 0.83 (t, J = 7.0 Hz, 6H), 0.77 (br, 4H). 13C{1H} NMR(150 MHz, CDCl3): δ 151.6, 145.4, 144.9, 143.8,
143.3, 141.5, 129.2, 126.1, 125.0, 123.1, 120.4, 105.9, 55.7, 40.2, 31.8, 29.9, 29.2, 23.8, 22.6, 14.0. 19F
NMR (376 MHz, CDCl3) : δ -135.0 (br, 4F), -139.0 (br, 4F). Anal. calcd. for C41H40F8: C 71.92, H 5.89; found
C 71.88, H 6.14.
3
Figure S1. 13C{1H} NMR spectrum of Polymer 1 that was synthesized via sequential protocol (Scheme 3
(a)) (150 MHz, in CDCl3).
Figure S2. MALDI-TOF-MS spectrum of Polymer 1 that was synthesized via sequential protocol (Scheme
3 (a)).
4
Figure S3. Absorption spectra of Polymer 1 in the film state. (a) Synthesized via sequential protocol
(Scheme 3 (a)) and (b) synthesized from the isolated 2,7-dibromo-9,9-dioctylfluorene (Scheme 2, Table
1, Entry 1).
Figure S4. GPC profile of Polymer 1 (Scheme 3 (a)).
5
Figure S5. 1H NMR spectrum of Polymer 2 that was synthesized via sequential protocol (Scheme 3 (b))
(400 MHz, in CDCl3).
Figure S6. 13C{1H} NMR spectrum of Polymer 2 that was synthesized via sequential protocol (Scheme
3(b)) (150 MHz, in CDCl3).
6
Figure S7. 19F NMR spectrum of Polymer 2 that was synthesized via sequential protocol (Scheme 3(b))
(377 MHz, in CDCl3).
Figure S8. MALDI-TOF-MS spectrum of Polymer 2 that was synthesized via sequential protocol (Scheme
3 (b)).
7
Figure S9. Absorption spectra of Polymer 2 in the film state. (a) Synthesized via sequential protocol
(Scheme 3 (b)) and (b) synthesized from the isolated 2,7-dibromo-9,9-dioctylfluorene.S5
Figure S10. GPC profile of Polymer 2 (Scheme 3 (b)).
References
[S1] Kajigaeshi, T. Kakinami, M. Moriwaki, T. Tanaka, S. Fujisaki, and T. Okamoto, Bull. Chem. Soc. Jpn.
1989, 62, 439.
[S2] a) Y. Zou, A. Najari, P. Berrouard, S. Beaupré , B. Réda Aïch, Y. Tao, M. Leclerc, J. Am. Chem. Soc.
2010, 132, 5330; b) T. E. Kang, H.-H. Cho, C.-H. Cho, K.-H. Kim, H. Kang, M. Lee, S. Lee, B. Kim, C. Im, B. J.
Kim, Appl. Mater. Interfaces 2013, 5, 861.
8
[S3] C. Xia, R. C. Advincula, Macromolecules 2001, 34, 5854
[S4] J. Kuwabara, K. Yamazaki, T. Yamagata, W. Tsuchida, T. Kanbara, Polym. Chem. 2015, 6, 891.
[S5] W. Lu, J. Kuwabara, T. Iijima, H. Higashimura, H. Hayashi, T. Kanbara, Macromolecules 2012, 45,
4128.
9