Hemocytometer
advertisement

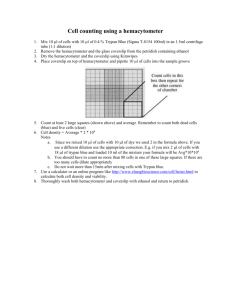
Lab 4: Hemocytometer The hemocytometer is a device used to count cells. It was originally designed for the counting of blood cells. The Neubauer chamber is a thick crystal slide with the size of a glass slide. (30 x 70 mm and 4 mm thickness) It is constructed so that the distance between the bottom of the coverslip and the surface of the counting area of the chamber is 0.1 mm. The surface of the chamber contains two square ruled areas separated by an Hshaped moat. These two squares are identical, allowing the technologist to duplicate the cell count. Each has a total area of 9 mm2 (1 mm on each side). These squares are divided into nine primary squares with an area of 1mm2. The four corner primary squares are used when counting leukocytes. (If the WBC is very low, you would count all 9 squares.) These 4 large corner squares contain 16 smaller secondary squares, each with an area of 0.04 mm2. All 25 secondary squares of the center primary square are used to count 1 platelets, and each of these 25 squares is further divided into 16 smaller tertiary squares. The boundary lines of the central primary square are either double or triple. When the boundary line is double, all the cells within the square and those touching the innermost line are counted. If the boundary line is triple, all of the cells within the squares and those touching the middle line inward are counted. Hemacytometers and coverslips should meet the specifications of the National Bureau of Standards and are so marked by the manufacturer. A standardized coverslip should be used that has been ground to fit the specifics of the hemacytometer, ensuring a uniform depth and therefore a constant volume. A regular coverslip cannot be used. 2 The necessary elements to perform a cell count with Neubauer chamber are as follows: a) cellular dilution to measure b) hemocytometer, or Neubabuer chamber c) optical microscope d) cover glass e) pippette / micropippete with disposable tips. f) Dilution buffer / PBS (if needed) STEP 1. Sample preparation. Depending on the type of sample, a preparation of a dilution with a suitable concentration should be prepared for cell counting. WBCs Specimen EDTA-anticoagulated blood or capillary blood is preferred. Reagents, supplies and equipment 1. White blood cells count diluting fluid which may be one of the following: - Acetic acid 2% (v/v) in distilled water. - HCL 1% (v/v) in distilled water. - Turks' solution which is formed of: A. Glacial acetic acid 3 ml B. Crystal violet 1 ml C. 100 ml distilled water. 2. Thoma white pipette. 3 Procedure 1. Draw the blood up to 0.5 mark in the thoma pipette. 2. Wipe the outside of the capillary pipette to remove excess blood that would interfere with the dilution factor. 3. Holding the pipette almost vertical place into the fluid. Draw the diluting fluid into the pipette slowly until the mixture reaches the 11 mark, while gently rotating the pipette to ensure a proper amount of mixing. 4. Place the pipette in a horizontal position and firmly hold the index finger of either hand over the opening in the tip of the pipette, detach the aspirator from the other end of the pipette now the dilution of the blood is completed 5. Mix the sample for at least 3 minutes to facilitate hemolysis of RBCs. 6. Clean the hemacytometer and its coverslip with an alcohol pad and then dry with a wipe. 7. Before filling the chamber, discard the first four to five drops of the mixture on apiece of gauze to expel the diluent from the stem. 8. Carefully charge hemacytometer with diluted blood by gently squeezing sides of reservoir to expel contents until chamber is properly filled. 4 9. Mount the hemacytometer on the microscope and lower its condenser. RBCs Sample Whole blood using EDTA or heparin as anticoagulant. Capillary blood may also be used. Diluting fluid One of the following solutions may be used: 1. Isotonic saline: 0.85%sodium chloride (NaCl) in distilled water. 2. Hayam’s solution A. Sodium Sulphate 10 g. B. Sodium Chloride 2 g. C. Mercuric Chloride 0.25 g. D. Distilled Water 100ml 3. Gower’s solution A. Sodium Sulphate 12.5 g. B. Glacial acetic acid 33.3 ml C. Distilled water 100 ml. 4. Citrate-formalin solution A. Tri-sodium Citrate B. Formalin Equipments (Pipettes) used one of the following: A. Thoma pipette (RBCs) B. Micropipette –20l is the desired volume. 5 Procedure 1. Dilution of the blood: Micropipette (20) 1:201 dilution. Pipette 4.0ml of diluting fluid into a tube Pipette 20l of will mixed anticoagulated whole blood to the tube. Thoma red count pipette. Draw the blood up to exactly the 0.5 mark and dilute to the 101 mark. Mix continuously for 2-3 minutes. 2. Load the cleaned hematocytometer. 3. Place the hematocytometer on the microscope stage, lower the condenser. 4. Focus with x10 objective lens on the large central square. This square is ruled into 25 small squares, each of which is further divided into 16 smaller squares, of the 25 squares, only the four corner squares, and one middle square are used to count RBCs. 5. Switch to 40 objective lens, and start counting in the five designated squares. STEP 2. Microscope set up and focus. 1. Place the Neubauer chamber on the microscope stage. If the microscope has a fixing clamp, fix the Neubauer chamber. 2. Turn on the microscope light. 3. Focus the microscope until you can see a sharp image of the cells looking through the eyepiece and adjusting the stage 4x, 10x, and 40 x. 4. Look for the first counting grid square where the cell count will start. 6 7 Calculation WBCs for counting 4 squares : Total WBC/mm3 = No. of cells x volume correction factor x dilution factor. Total WBC/mm3 = No. of cells x (10/4) x 20. Correction for dilution: The thoma pipette is 1:20 Dilution factor 20 Correction of volume: Volume of square: One large square is = 0.1 x 1 x 1= 0.1 μL. Volume of 4 squares: Four large squares are 0.4 μL. Volume correction factor: 1/0.4 RBCs Total RBC Count = N x Dilution Factor x Volume Correction Factor Where: N = the total number of red cells counted in the counting chamber. Dilution 1: 200 Dilution Factor = 200 Counted Volume Each counted square has a volume of 0.2 X 0.2 X 0.1 = 0.004 5 squares volume = 5 X 0.004 = 0.02 cumm Volume correction factor = 1/0.02 = 50 So, Total RBC count = N X 200 X 50 = N X 10.000 8