Limiting Reagent Lab - Hrsbstaff.ednet.ns.ca
advertisement

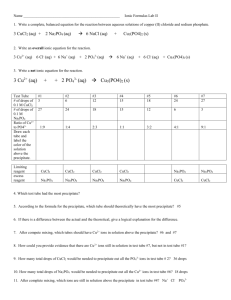
Chemistry 11 Formula of a Hydrate Lab Background Do all chemical reactions occur in exact proportions? What happens if there is extra (leftover) reactants (reagents)? How will you observe this? How can we quantify it? We will examine the following reaction: 2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu(s) Useful Information Copper (II) chloride will be used in its hydrated form (CuCl2 . 2H2O, copper (II) chloride dihydrate) initially. This means that when beginning a mass-mass problem you will need to use the molar mass of CuCl2 . 2H2O, it will be approximately 170 g/mol. Also of interest when CuCl2 is dissolved in water it releases the Cu2+ ion, this is blue in color. Aluminum in its elemental form is a shiny grey metal that does not dissolve in water. If it “dissappears” it means that it has reacted. Safety Copper (II) chloride is corrosive and a health hazard. 4 M hydrochloric acid is very strong and quite corrosive, please do not remove it from the fume-hood. Wear goggles the entire time you are in the lab and as always wash your hands before eating anything. Materials CuCl2 . 2H2O(s) 4 M HCl(aq) aluminum foil (Al(s)) glass stir rod 250 mL beaker Procedure 1. Obtain a piece of masking tape and a marker and label a dry 250 mL beaker. 2. Record the mass of the dry (labelled) 250 mL beaker here:____________________. 3. Mass 1.5 - 2 g of CuCl2 2H2O solid into the 250 mL beaker. The amount is up to you but record the exact mass to two decimal places. 4. Add 100 mL of water to the CuCl2, stir with the glass rod until it is dissolved. 5. Cut off a strip of aluminum foil, ensure the mass is between 0.10 and 0.15 g, recording the exact mass. 6. Loosely fold then place the strip in the beaker of CuCl2, add ~1 mL of HCl and stir gently with the rod. 7. Record your observations. 8. When the reaction appears to be complete (how will you assess this?), allow the product to settle. 9. Pour off the excess liquid (this is known as decanting). 10. Wash the product with 20 mL of water, decant again. 11. Allow the product to dry in the oven. 12. Complete the write-up on a separate piece of paper and submit it individually. Data and Analysis Please record your data and observations below. 1. Mass of CuCl2 . 2H2O(s) in grams. __________________________ 2. Mass of Al(s) in grams. __________________________ 3. Mass of product, Cu(s), collected once dry in grams. __________________________ Data and Analysis (continued…) Please record your data and observations below. 4. Use your mass of CuCl2 . 2H2O as a starting point and perform a mass-mass calculation for Cu(s). 5. Use your mass of Al(s) as a starting point and perform a mass-mass calculation for Cu(s). 6. Only one of the answers from 4 and 5 can be the “correct” mass of Cu(s) to form. Which is it? Explain. 7. Use your mass of CuCl2 . 2H2O as a starting point and perform a mass-mass calculation for Al(s). 8. Compare your answer for question 7 to the actual amount of Al(s) you had in the experiment. Which is greater? 9. Did you have enough Al(s) to react with the amount of CuCl2 . 2H2O you used? 10. What would you expect to observe if some more Al(s) were added to the reaction mixture before you decanted? 11. The reactant (reagent) that “runs out” first is known as the limiting reagent. Whichever reagent gave the smaller answer for questions 4 and 5 is known as the limiting reagent. The smaller answer from questions 4 and 5 is known as the theoretical yield (ie how much copper should form over the course of the reaction. The experimental yield is what was obtained experimentally, you wrote this as number 3 above. 12. Use your values from this lab to determine your percent yield.