Stoichiometry of Precipitates
advertisement

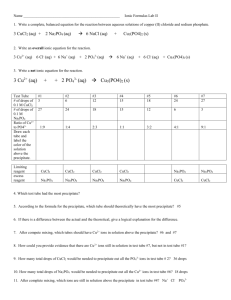
Stoichiometry of PrecipitatesII 1) Purpose: To carry out a double replacement reaction and isolate the precipitate, confiming reaction Stoichiometry. 2) Theory: Given the reaction: 3Zn(NO3)2(aq) + 2Na3PO4(aq) Zn3(PO4)2(cr) + 6Na NO3(aq) This reaction is slightly exothermic and poses no danger thermally. Zinc Nitrate is toxic and needs to be treated with respect. Zinc Phosphate will be precipitated as a finely divided solid that will quickly flocculate (clump together) and sink to the bottom os your beaker. It can be separated out by decantation and filtration, dried and massed to confirm reaction stoichiometry and % yield. 3) Procedure: Base all calculations using 1.0 grams of anhydrous Sodium Phosphate as the limiting reagent. Use a 200% excess of Zinc Nitrate. Keep in mind that both these compounds are Hydrates and you need to convert hydrate mass to anhydrous mass. Nitrates are notoriously hygroscopic and rarely are in their normal hydrate state. A sample calculation would be: 2g Na3PO4 X 1mol Na3PO4/164g Na3PO4 X 1mol Na3PO4*12H2O/1mol Na3PO4 X 380g Na3PO4*12H2O/1mol Na3PO4*12H2O= grams Na3PO4*12H2O to weigh out Dissolve both chemicals in two separate beakers (stirring helps!). watch out for contamination. After dissolving, mix the two solutions together and allow the precipitate to flocculate and sink to the bottom. Decant off the excess clear solution, then gravity filter the slurry (don’t forget to mass the filter paper before you filter!!). Rinse the slurry 5 times with dionized water. Remove the filter paper, keeping the filter cake intact, and place it in a small labelled beaker and dry in the oven for 24 hours. 4) Error: Think carefully about the order of importance of error sources in this lab. Feel free to ask questions in class. 5) Conclusion: As discussed in class, your percent yield is your absolute error. How did you do? How pure do you think your product is? Can you back up your estimate of purity? How good is your yield in the context of your relative error?