EXTENSION: The Wave Nature of Matter
advertisement

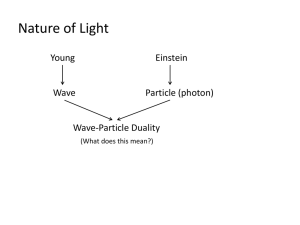
EXTENSION: Wave/Quantum Mechanics, Orbitals and Electron Probability Density Review de Broglie hypothesized that an electron could behave like a _______(1923) de Broglie hypothesis received experimental support in 1927 Shrodinger, inspired by de Broglie’s idea of __________ _________, Einstein’s idea of ________energy particles (_________), and *Heisenberg’s proposed uncertainty principle, Schrodinger developed a branch of physics called wave mechanics, often referred to as ______________ mechanics. Quantum mechanics is the current theory of atomic structure. This model describes atoms as having certain allowed quantities of energy because of the wave-like properties of their electrons. Recall: Shrodinger used wave equations to predict the probability of finding an electron at a particular point in an atom. These wave equations are called orbitals. *Heisenberg Uncertainty Principle (1927) Using mathematics, Heisenberg showed that it is _________ to show exactly where an electron is or how fast it is traveling. *Electron Probability Density a mathematical or graphical representation of the chance of finding an electron in a given space (see pp. 200-201) EXTENSION The Wave Nature of Matter (Website) http://www.colorado.edu/physics/2000/quantumzone/debroglie.html: *Sometimes light displays particle-like behavior, and sometimes it acts like a wave; it all depends on what sort of experiment you're doing. This is known as wave/particle duality, and, like it or not, physicists have just been forced to accept it. *If we begin to think of electrons as waves, we'll have to change our whole concept of what an "orbit" is. Instead of having a little particle whizzing around the nucleus in a circular path, we'd have a wave sort of strung out around the whole circle. Now, the only way such a wave could exist is if a whole number of its wavelengths fit exactly around the circle. If the circumference is exactly as long as two wavelengths, say, or three or four or five, that's great, but two and a half won't cut it. So there could only be orbits of certain sizes, depending on the electrons' wavelengths --which depend on their momentum. *If electrons are waves, then it makes sense that they don't give off or absorb photons unless they change energy levels. If it stays in the same energy level, the wave isn't really orbiting or "vibrating" the way an electron does in Rutherford's model, so there's no reason for it to emit any radiation. And if it drops to a lower energy level,, the wavelength would be longer, which means the frequency would decrease, so the electron would have less energy. (*E=hf) Then it makes sense that the extra energy would have to go someplace, so it would escape as a photon--and the opposite would happen if a photon came in with the right amount of energy to bump the electron up to a higher level. 1