Heating and Microwave assisted SPPS of C
advertisement

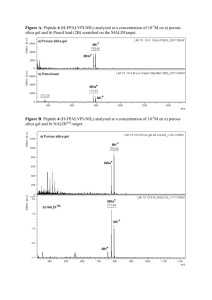
Heating and Microwave assisted SPPS of C-terminal acid peptides on trityl resin: The truth behind the yield Cécile Echalier a†• Soultan Al-Halifaa • Aude Kreiter†• Christine Enjalbal • Pierre Sanchez • Luisa Ronga †• Karine Puget †• Pascal Verdié • Muriel Amblard • Jean Martinez • Gilles Subra Table of Contents Experimental Conditions Analytical HPLC analyses S2 LC/MS analyses S2 RP-Preparative HPLC S2 First amino acid anchoring on hydroxymethyl phenoxy PS resin (Wang) S3 First amino acid anchoring on Fmoc-Rink amide aminomethyl PS S3 resin Synthesis of compound 8 c[Phe-Ala] DKP S3 Titration of Fmoc-Ala-OH 4’ (HPLC =214 nm) S5 Calibration curve of Fmoc-Ala-OH 4’ S5 Titration of released Fmoc-Ala-OH from the resin at room temperature in DMF S5 Titration of released Fmoc-Ala-OH from the resin at 70°C in DMF S6 Titration of Fmoc-Phe-Ala-OH 5’ (HPLC =214 nm) S6 Calibration curve of peptide 5’ S6 Titration of released peptide 5’ from the resin at room temperature in DMF S7 Titration of released peptide 5’ from the resin at 70°C in DMF S7 Titration of Fmoc-Leu-Phe-Ala-OH 7’ (HPLC =214 nm) S8 Calibration curve of peptide 7’ S8 Titration of released peptide 7’ from the resin at room temperature in DMF S8 Titration of released peptide 7’ from the resin at 70°C in DMF S9 Calibration curve of DKP 8 (LC/MS, SIR MRM m/z 219.2) S9 Calibration curve of protected peptide 1’ (HPLC =214 nm) S10 LC/MS analyses of crude peptide 1’ S10 LC chromatogram of purified peptide 1’ S11 Titration of released protected peptide 1’ S12 LC/MS analyses of Crude Peptide 1 H-Glu-Lys-Arg-Tyr-Cys-Ser-OH S13 Determination of stereochemistry of released peptide from resin 7 S15 Synthesis scheme of R2 with resin weighting and calculations S17 Synthesis scheme of R3 with resin weighting and calculations S17 LC/MS Analysis of peptide 2 S18 S1 LC/MS Analysis of peptides 3 S19 Analytical HPLC analyses Conditions A Analytical HPLC were run on a Beckman instrument, equipped with a photodiode array and Chromolith SpeedROD (50 – 4.6 mm) reversed-phase column (RP-18e). Standard conditions were eluent system A (water/0.1% TFA), system B (acetonitrile/0.1% TFA). A flow rate of 5 mL/min and a gradient of (0–100)% B over 3 min were used, detection at 214 nm, unless otherwise stated. Retention times (Rt(A)) are given in minutes. Conditions B Analytical HPLC were run on an Agilent 1100 instrument, equipped with a photodiode array and XBridge (150 – 3 mm) reversed-phase column (C18). Standard conditions were eluent system A (H2O/ACN/MSA 98/2/0.1%), system B (ACN/H2O/MSA - 98/2/0.1%). A flow rate of 5 mL/min and a gradient of (0–50)% B for 2 min, (50–100)% B for 5 min were used, detection at 214 nm, unless otherwise stated. Retention times (Rt(B)) are given in minutes. Conditions C LC analyses were run on a Waters Alliance 2695 HPLC, equipped with monochromatic detector array and Chromolith High resolution (25 – 4.6 mm) reversed-phase column (RP-18e). A flow rate of 3 mL/min and a gradient of (0–100) % B over 2.5 min (or over 15 min) were used. Eluent A: water/0.1% HCO 2H; eluent B: acetonitrile/0.1% HCO2H. Retention times (Rt(C)) are given in minutes Conditions D (for separation of diastereoisomers of 7’) LC analyses were run on a Beckman instrument, equipped with a monochromatic detector and Onyx monolithic HD (50 – 4.6 mm) reversed-phase column (C18). Standard conditions were eluent system A (water/0.1% TFA), system B (acetonitrile/0.1% TFA). A flow rate of 5 mL/min and a gradient of (32–34)% B over 30 min were used, detection at 214 nm. Retention times (Rt(D)) are given in minutes. LC/MS analyses LC/MS analyses – samples were prepared in acetonitrile/water (50/50 v/v) mixture, containing 0.1% TFA. The LC/MS system consisted of a Waters Alliance 2695 HPLC, coupled to a Micromass (Manchester, UK) ZQ spectrometer (electrospray ionization mode, ESI+). All the analyses were carried out using a Merck Chromolith Speed rod C18, 25 × 4.6 mm reversed-phase column. A flow rate of 3 mL/min and a gradient of (0–100) % B over 2.5 min (or over 15 min) were used. Eluent A: water/0.1% HCO 2H; eluent B: acetonitrile/0.1% HCO2H. Retention times (RT) are given in minutes. Positive ion electrospray mass spectra were acquired at a solvent flow rate of 200 mL/min. Nitrogen was used for both the nebulizing and drying gas. The data were obtained in a scan mode ranging from 100 to 1000 m/z in 0.1 s intervals; 10 scans were summed up to get the final spectrum. MRM analysis of compound 8 c[Phe-Ala] S2 Diketopiperazine c[Phe-Ala] analysis was perform using MRM acquisition mode (multiply reaction monitory) on a Waters Alliance 2695 coupled to a Micromass Quatro Micro. A flow rate of 3 mL/min and a gradient of (0– 100) % B over 2.5 min were used. Eluent A: water/0.1% HCO2H; eluent B: acetonitrile/0.1% HCO2H. Retention times (RT) are given in minutes. Detection of DKP was done at 214 nm using the same acquisition (ES+) and the following parameters: Cone voltage: 20 V; Collision energy: 12 eV. RP-Preparative HPLC RP-preparative HPLC purification was performed on a Waters HPLC 4000 instrument, equipped with a UV detector 486 and Waters Delta-Pack 40×100 mm, 15 Å, 100 µm, reversed-phase column. Eluent system A (water/0.1% TFA), system B (acetonitrile/0.1% TFA). A flow rate of 50 mL/min and a gradient of (10–70)% B over 30 min were used, detection at =214 nm. First amino acid anchoring on hydroxymethyl phenoxy PS resin (Wang), example of Fmoc-Ala-OH. The hydroxymethyl phenoxy PS resin (1g, 0.7 mmol, initial theoretical loading = 0.7 mmol/g) was conditioned for 15 min in DCM. Then, 10 mL of DMF solution of Fmoc-Ala-OH (3 eq., 2.1 mmol, 654 mg) containing DIC (1.5 eq, 1.05 mmol, 165 µl), DMAP (0.15 eq, 105 µmole, 13 mg) was poured unto the resin and stirred overnight. The resin was filtered and then washed with DMF (2×), DCM/MeOH 1/1 v/v (2×), DMF (2×), DCM (2×) and dried in a desiccator overnight. First amino acid anchoring on Fmoc-Rink amide aminomethyl PS, example of Fmoc-Ala-OH. The Fmoc rink amide aminomethyl PS resin (1g, 0.96 mmol, initial theoritical loading 0.96 mmol/g) was conditioned for 15 min in DCM. The Fmoc protecting group was removed with a standard deprotection cycle using 80/20 v/v DMF/pip solution (see main article Material and Methods). After washing step, the first aminoacid was loaded on the resin through a standard coupling cycle (see main article Material and Methods) using HBTU and NMM. Synthesis of compound 8 c[Phe-Ala] DKP. (3R,6S)-3-benzyl-6-methylpiperazine-2,5-dione O O N H OH O HBTU (1.1 eq) NMM (2 eq) NH2 O + O O DMF, RT 2hours O O H N N H O O TFA DCM (3R,6S)-3-benzyl-6-methylpiperazine-2,5-dione O DCM reflux HN DIEA (10 eq) 6 hours NH 8 O S3 H N H2N O O O Boc-Ala-OH (1.1 eq) was dissolved in a solution of HBTU (1.1 eq), N-Methylmorpholine (NMM; 2 eq) in N,Ndimethylformamide (DMF), and the resulting solution was added to the H-Phe-OMe dissolved in DMF. The reaction mixture was stirred at room temperature for 2 h and then DMF was evaporated under vacuum. The residue was dissolved in AcOEt and washed with KHSO4 (3 times), NaHCO3 (3 times), NaCl (3 times) and then dried over anhydrous sodium sulfate and evaporated to give an oily residue (compound 1). The compound 1 was dissolved in trifluoroacetic acid (TFA) and stirred for 1 hour, followed by removal of the solvent by evaporation under vacuum to give compound 2. This compound 2 was refluxed in DCM for 6 hours and DKP (compound 8) precipitates directly into the flask. The precipitate was washed with DCM, Et 2O, frozen and lyophilized in water/acetonitrile (50/50). MS (ESI) calcd for C12H14N2O2, 218.11; [M + H]+m/z found 219.20 , Rt(C): 0.82 min. TIC/LC and LC/MS analyses are presented in the following figures S4 Titration of Fmoc-Ala-OH 4’ (HPLC =214 nm) Calibration curve of Fmoc-Ala-OH 4’ A solution of Fmoc-Ala-OH (stock solution, 10-3 M) was prepared in 4 mL of DMF. Stock solution was used to make a dilution range of 10-3 M to 10-6 M and 10 µl of each solution was injected on an analytical HPLC (conditions A). Data are summarized in the following table. Titration (M) 0 6.2x10-5 7.75x10-5 1.03x10-4 1.55x10-4 Areas 0 261479 453235 543635 730797 S5 HPLC 214nm Peak area= f[Fmoc-Ala-OH] 800000 y = 4.96E+09x R² = 9.83E-01 600000 400000 200000 Conc (M) 0 0.00E+00 5.00E-05 1.00E-04 1.50E-04 2.00E-04 Titration of released Fmoc-Ala-OH from the resin at room temperature in DMF 100 mg of resin 4 were weighted and placed in a test tube. 4 mL of DMF were added and kinetics was carried out at room temperature on an analytic HPLC (Conditions A) for 1200 min. Data are summarized in the following table. Time (min) 0 60 150 240 360 1200 Area 73117 90434 298272 271477 199759 203332 Titration (M) 1.25 x10-5 1.61 x10-5 5.89 x10-5 5.23 x10-5 3.86 x10-5 3.93 x10-5 HPLC 214 nm [Fmoc-Ala]= f(time) 1.00E-04 8.00E-05 6.00E-05 4.00E-05 2.00E-05 5.00E-19 0 500 1000 1500 Time (min) -2.00E-05 Titration of released Fmoc-Ala-OH from the resin at 70°C in DMF In a closed vessel containing 100 mg of resin 4, 4 mL of DMF were added and the mixture was heat till 70°C. Kinetics was carried out on an analytic HPLC (conditions A) for 400 min. Data are summarized in the following table. Time (min) 0 60 150 240 400 Area 11407110 32217440 49795400 11987300 0 Titration (M) 2.349 x10-3 6.640 x10-3 1.026 x10-3 2.469 x10-3 0 S6 HPLC 214 nm [Fmoc-Ala-OH] = f(time) 1.20E-02 1.00E-02 Turbi dirty 8.00E-03 6.00E-03 4.00E-03 2.00E-03 Time (min) 0.00E+00 0 100 200 300 400 500 Titration of Fmoc-Phe-Ala-OH 5’ (HPLC =214 nm) Calibration curve of peptide 5’ A solution of Fmoc-Phe-Ala-OH (stock solution, 10-3 M) was prepared in 4 mL of DMF. Stock solution was used to make a dilution range of 10-3 M to 10-5 M and 10 µl of each solution was injected on an analytical HPLC (Conditions A). Data are summarized in the following table. Titration (M) 2.10-5 1.10-4 2.10-4 3.10-4 4.10-4 Areas 43035 237928 469037 797828 963521 HPLC 214 Peak area = f[Fmoc-Phe-Ala-OH] 1200000 1000000 800000 y = 2,46E+09x R² = 9,92E-01 600000 400000 200000 0 0.00E+00 Conc (M) 1.00E-04 2.00E-04 3.00E-04 4.00E-04 5.00E-04 Titration of released peptide 5’ from the resin at room temperature in DMF 100 mg of resin 5’ were weighted and placed in a test tube. 4 mL of DMF were added and kinetics was carried out at room temperature on an analytic HPLC (Conditions A) for 400 min. Data are summarized in the following table. Time (min) 0 Area 34564 Titration (M) 1.41 x10 67 132 184223 -5 7.49 x10 -5 206 243800 9.91 x10 S7 315 215285 -5 8.75 x10 -5 388 229580 9.33 x10 258306 -5 1.05 x10-4 HPLC 214 nm [Fmoc-Phe-Ala-OH] = f(time) 1.20E-04 1.00E-04 8.00E-05 6.00E-05 4.00E-05 2.00E-05 Time(min) 0.00E+00 0 100 200 300 400 500 Titration of released peptide 5’ from the resin at 70°C in DMF In a closed vessel containing 100 mg of resin 5’, 4 mL of DMF were added and the mixture was heat till 70°C. Kinetics was carried out on an analytic HPLC (Conditions A) for 400 min. Data are summarized in the following table. Time (min) 5 75 137 215 302 390 Area 691751 3676338 1353920 2037955 1669751 1303901 Titration (M) 2.81 x10-4 1.49 x10-3 5.50 x10-3 8.28 x10-3 13.6 x10-3 15.9 x10-3 HPLC 214 nm [C] = f[time] 1.80E-02 1.60E-02 1.40E-02 1.20E-02 1.00E-02 8.00E-03 6.00E-03 4.00E-03 2.00E-03 Time (min) 0.00E+00 0 100 200 300 400 500 Titration of Fmoc-Leu-Phe-Ala-OH 7’ (HPLC =214 nm) Calibration curve of peptide 7’ A solution of Fmoc-Phe-Ala-OH (stock solution, 10-2 M) was prepared in 4 mL of DMF. Stock solution was used to make a dilution range of 10-2M to 10-5 M and 10 µl of each solution was injected on an analytical HPLC (Conditions A). Data are summarized in the following table. Titration (M) Areas 1.67 x10-4 3.33 x10-4 6.66 x10-4 1 x10-3 1.5 x10-3 2.25 x10-3 3.38 x10-3 5.07 x10-3 76041 174998 352007 626643 829560 1180207 1730425 2417291 S8 HPLC 214 nm Peak area = f[Fmoc-Leu-Phe-Ala-OH] 3000000 2500000 y = 4.98E+08x R² = 9.91E-01 2000000 1500000 1000000 500000 Conc (M) 0 0.00E+00 1.00E-03 2.00E-03 3.00E-03 4.00E-03 5.00E-03 6.00E-03 Titration of released peptide 7’ from the resin at room temperature in DMF 100 mg of resin 7’ were weighted and placed in a test tube. 4 mL of DMF were added and kinetics was carried out at room temperature on an analytic HPLC (Conditions A) for 400 min. Data are summarized in the following table. Time (min) 0 65 129 209 313 391 Area 34564 184223 243800 215285 229580 258306 Titration (M) 1.20 x10-5 1.14 x10-4 1.35 x10-4 1.42 x10-4 1.75 x10-4 1.64 x10-4 HPLC 214 nm [Fmoc-Leu-Phe-Ala-OH] = f[time] 2.00E-04 1.50E-04 1.00E-04 5.00E-05 Time (min) 0.00E+00 0 100 200 300 400 500 Titration of released Peptide 7’ from the resin at 70°C in DMF In a closed vessel containing 100 mg of resin 5’, 4 mL of DMF were added and the mixture was heat till 70°C. Kinetics was carried out on an analytic HPLC (Conditions A) for 400 min. Data are summarized in the following table. Time (min) 10 89 149 225 318 400 Area 1109903 3631953 2184003 1135121 1441678 852544 Titration (M) 3.4 x10-4 1.12 x10-3 3.38 x10-3 7.03 x10-3 8.93 x10-3 7.92 x10-3 S9 HPLC 214 nm [Fmoc-Leu-Phe-Ala-OH] = f(time) 1.00E-02 8.00E-03 6.00E-03 4.00E-03 2.00E-03 Time (min) 0.00E+00 0 100 200 300 400 500 Calibration curve of DKP 8 (LC/MS, SIR MRM m/z 219.2) A solution of DKP (stock solution, 10-2 M) was prepared using 2.18 mg of DKP in 1 mL of water/acetonitrile (50/50). Stock solution was used to make a dilution range of 10 -2 M to 5.10-6 M and 1 µl of each solution was injected on a mass spectrometer (Mass lynx power). Data are summarized in the following table. Titration (M) 1 x10-3 5 x10-4 1 x10-4 5 x10-5 1 x10-5 5 x10-6 areas 7859 3700 791 374.21 59 51.54 HPLC 214 nm Peak area = f[DKP] 8000 y = 8E+06x R² = 0,9991 6000 4000 2000 0 0.00E+00 Conc (M) 5.00E-04 1.00E-03 1.50E-03 Calibration curve of protected peptide 1’ (HPLC =214 nm): Boc-Glu(OtBu)-Lys(Boc)-Arg(Pbf)-Tyr(tBu)Cys(trt)-Ser(tBu)-OH MW = 1646.8 g/mol. Protected peptide 3 was synthesized on a LibertyTM Microwave Peptide Synthesizer (CEM Corporation, Matthews, NC) providing MW irradiation at 2450 MHz using Fmoc/tBu SPPS strategy in the conditions described in Material and methods section of the article. The crude peptide 3 was released from the resin using a solution of HFIP for 45 min in order to keep the side chain protecting groups. LC/MS (conditions C) analyses are presented below. S10 [M-trt+H]+ [M+H]+ [M+H+Na]2+ [M+2Na]2+ [M-trt+Na]+ [M+Na]+ The crude peptide was purified on an autopurification system Waters Micromass equipped with an injector/collector sample manager (Waters 2767), a pump (Waters 2767), a photodiode array detector (Waters 2996) and a mass spectrometer ZQ (Waters). LC chromatogram (conditions C) of purified 1’ is presented below. S11 A calibration curve of the pure peptide (stock solution, 10-3 M) was prepared in 4 mL of DMF. Stock solution was used to make a dilution range of 10-3M to 10-6 M and 10 µl of each solution was injected on an analytical HPLC (Conditions B). Data are summarized in the following table. Titration (M) 7.80 x10-6 1.56 x10-5 3.12 x10-5 6.25 x10-5 1.25 x10-4 2.50 x10-4 5.00 x10-4 7.60 x10-4 Area 514.5 562.1 746.9 1046 1697 2625 4725 6543 HPLC 214 Peak area = f[Protected peptide 1'] 8000 y = 9E+06x R² = 0,9589 6000 4000 2000 0 0.00E+00 Conc (M) 2.50E-04 5.00E-04 7.50E-04 1.00E-03 Titration of released Peptide 1’ from the resin at 70°C in DMF In a closed vessel containing 100 mg of resin R1’, 4 mL of DMF were added and the mixture was heat till 70°C. Aliquots were analyzed (conditions A) for 400 min. Data are summarized in the following table. Time (hour) Peak area Titration (M) S12 1 1678 2.80 x10-4 2 2727 4.60 x10-4 3 3929 6.60 x10-4 4 6791 1.10 x10-3 5 4001 1.35 x10-3 6 4313 1.45 x10-3 7 4853 1.60 x10-3 8 3161 1.60 x10-3 9 4424 2.20 x10-3 10 3055 2.10 x10-3 11 3257 2.20 x10-3 12 3896 2.60 x10-3 13 4173 2.80 x10-3 14 4517 3.00 x10-3 15 4628 3.10 x10-3 16 4086 3.40 x10-3 17 4050 3.40 x10-3 18 4752 4.00 x10-3 19 5293 4.50 x10-3 LC/MS analysis of Crude Peptide 1 H-Glu-Lys-Arg-Tyr-Cys-Ser-OH, MW = 784.8 g/mol S13 [M+2H]2+ [M+H]+ S14 Determination of stereochemistry of released peptide from resin 7 Chromatogram of 7’ Fmoc-Leu-Phe-Ala-OH (conditions D) Fmoc H N O N H H N O (S) OH O Chromatogram of 7’(D) Fmoc-Leu-Phe-(D)Ala-OH (conditions D) Fmoc H N O N H H N O (R) OH O S15 Coinjection of 7’(D) and 7’ (conditions D) 7’ Fmoc H N O N H H N 7’(D) O (S) OH O Fmoc Released peptide from resin 7 after 6 hours heating (70°C) Dibenzofulvene Released peptide DMF S16 H N O N H H N O O (R) OH Coinjection of released peptide after 6 hours heating (70°C) and 7’(D) (Fmoc-Leu-Ala-(D)Phe-OH) 7’(D) (Fmoc-Leu-Ala-(D)Phe-OH) Released peptide Dibenzofulvene S17 Synthesis scheme of R2 with resin weighting and calculations starting resin R2 0.76 mmol/g 0.1 mmol scale 2 batches (a and b) of 132 mg R2' Fmoc SPPS Fmoc H N O H-Gly-Ser(tBu)-Asn(trt)-Lys(Boc)-Gly-Ala-Ile-Ile-Gly-Leu-Met O O Cl a: MW 70°C or b: RT S 0.1 mmol scale MW increment / resin R2= +1087.6 theoretical weight : 240.7 mg R2'a: 100.9 mg R2'b: 226.0 mg TFA/TIS/H2O 95/2.5/2.5 v/v/v 1 hour, RT H-Gly-Ser-Asn-Lys-Gly-Ala-Ile-Ile-Gly-Leu-Met-OH 2 peptide 2 weight 0.1 mmol scale theoretical :106.0 mg 1a: 2.8 mg 1b: 100.8 mg Synthesis scheme of R3 with resin weighting and calculations starting resin R3 0.72 mmol/g 0.1 mmol scale 2 batches (a and b) of 139 mg R3' Fmoc SPPS Fmoc H N O O H-Trp(Boc)-His(trt)-Trp(Boc)-Leu-Gln(trt)-Leu-Lys(Boc)-Pro-Gly-Gln(trt)-Pro-Met-Tyr(tBu) O Cl a: MW 70°C or b: RT O 0.1 mmol scale MW increment / resin R3= +2308g/mol theoretical weight : 369.8 mg R3'a: 365.0 mg R3'b: 94.2mg TFA/TIS/H2O 95/2.5/2.5 v/v/v 1 hour, RT H-Trp-His-Trp-Leu-Gln-Leu-Lys-Pro-Gly-Gln-Pro-Met-Tyr-OH 3 peptide 2 weight 0.1 mmol scale theoretical 168.4mg 1a: 0.8 mg 1b: 152.2 mg S18 LC/MS analysis of peptide 2b [M+H]+ [M+2H]2+ S19 LC/MS analysis of peptide2+3b [M+2H] [M+2H]2+ [M+2H]2+ S20