SCH 4C Name: Unit 1: Matter Date: Qualitative Chemistry Lab
advertisement

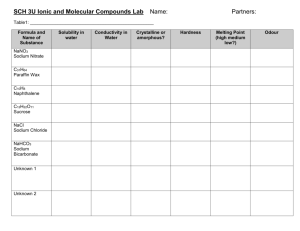
SCH 4C Unit 1: Matter Qualitative Chemistry Name: ___________________________ Date: ___________________________ Lab: Classifying Solids Using Qualitative Physical Properties Introduction: Recall that molecules are made of only non-metals that share valence electrons. This sharing of electrons is called a covalent bond. Ionic compounds are held together by ionic bonds. These bonds are formed between positively and negatively charged ions. Ionic compounds may be made up of the ions of a metal and a non-metal, a metal and a polyatomic ion or two polyatomic ions. Because they are held together differently, molecules and ionic compounds have different qualitative physical properties. Here is a table showing some of these differences. Conductivity Solubility in Water Hardness Melting point (Does the substance conduct (Will the substance of the solid of the solid electricity?) dissolve in water?) Ionic compounds will conduct Most ionic compounds Ionic solids are hard High – often 500oC or Ionic are at least a little bit and often brittle. higher. compound electricity when they are molecule dissolved in water (this makes them electrolytes) or are molten. Their ions cannot move in the solid state, though, so they cannot conduct electricity as solids. Acids are the only molecules that will conduct electricity when dissolved in water. This means that the majority of molecules do not conduct electricity. soluble in water. Some molecules are soluble in water, but there are a great many that are not. Different molecular solids have different amounts of hardness. Some are soft and some are really hard. The melting points of molecular solids are usually quite a bit less than for ionic compounds. Question How do ionic solids and molecular solids compare on the basis of conductivity, solubility in water, hardness and conductivity? Hypothesis: If ionic solids and molecular solids have different qualitative physical properties then ________________________ _____________________________________________________________________________________________ . Materials Goggles 4 small beakers Distilled water Sucrose Conductivity meter Potassium iodide Camphor Sodium chloride Stir stick Beaker for waste Method Part 1: Testing for Water Solubility 1. 2. 3. 4. 5. 6. 7. Gather enough small beakers so that there can be one different solid in each one. Use tape and a pencil to label the small beakers with the identity of one of the solids. Pour 10 mL of distilled water into each beaker. Add a small amount (just enough to fill up the end of a wooden splint) of each solid to its labelled beaker. Use a stirring rod to gently mix the contents of each beaker. Wipe the stirring rod with a piece of paper towel between beakers so you do not contaminate samples. Record whether or not each solid has dissolved in water in your observation table. You need the beakers and their contents for the next part of the activity so do not discard anything. Part 2: Testing for Conductivity 1. 2. Test each sample for electrical conductivity in the manner demonstrated by your teacher. Be sure to clean off the conductivity meter in distilled water before moving between beaker samples. Discard each solution by pouring it into the waste beaker provided. Part 3: Testing for Hardness 1. 2. 3. 4. 5. Use a wooden splint or a plastic spoon to place a few small crystals of one of the solids on a watch glass. Try to crush the crystals between the wooden splint and the watch glass. Record your results. Discard the crystals in the waste beaker. Repeat the above procedure for each of the other solids. Rank the solids from the one that was easiest to crush (call it #1) to the one that was hardest to crush (call it #4) in your observation table. Part 4: Melting Point Write the following melting points into your observation table. Potassium iodide, 686oC.; Sodium chloride, 801oC; camphor 177oC and sucrose 186oC. Part 5: Classifying an Unidentified Sample 1. Your teacher will provide you with an unknown sample. S/he will tell you the melting point. You need to test for the other qualitative physical properties of the substance and record them in your table. Observations: Property Solubility in Water Substance Potassium iodide Conductivity When in Water Hardness of the Solid Melting Temperature of the Solid Sodium chloride Camphor Sucrose (table sugar) Unknown Substance Analysis: Put the underlined title “Lab: Classifying Solids Using Qualitative Physical Properties” at the top of a piece of looseleaf. Put the title “Analysis” underneath that title. Answer each of the following questions in full and complete sentences. 1. 2. 3. 4. Identify whether each one of the four solids tested in parts 1 – 4 of the procedure is an ionic solid or a molecule. Use full sentences to explain your reasoning for EACH ONE. The table in the “Introduction” part of the lab will help you. Is the unknown substance a molecule or is it an ionic compound? Explain the reasoning for your choice. Although you do not have a record of the qualitative physical properties of the following substances, decide if each one is an ionic solid or a molecule based on its formula. [The first paragraph of the “Introduction” should help you to remember how to do this.] a) CH4 b) Li2SO4 c) NH4NO3 d) BaCl2 e) C2H3OH Here are the ways we represent the bonding in molecules and in ionic compounds. i) Molecules ii) Ionic compounds Use this information to draw out the identity of the ? for each of the following. [HINT: Use your answers from #3 above to help determine which substance is a molecule and which is an ionic compound before you do the drawings.] Conclusion: Write the underlined title “Conclusion” after your answers to the Analysis section. Write a few statements relating your hypothesis to what you found out in the lab.