Supplementary methods
advertisement

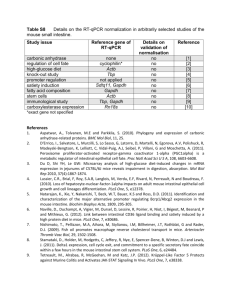
Supplementary methods Cell culture The human leukemic cell line K562 (ACC 10, DSMZ) and human prostate carcinoma PC3 cells (ACC 465, DSMZ) cells were respectively grown in RPMI 1640 medium with glutamax-I and in 45% Ham F12 + 45% RPMI 1640 (Life technologies) supplemented with 10% (vol/vol) fetal bovine serum. Flow cytometry The exposure of phosphatidyl-serine on the outer plasma membrane leaflet was identified using ANXA5/annexin V-FITC according to the manufacturer’s instructions (ANXA5/Annexin V-FITC apoptosis detection kit I, Pharmingen, 556420). For the cell cycle, cells were washed twice with ice-cold PBS and fixed overnight in 100% ethanol. Then, cells were washed twice with ice-cold PBS and incubated at 37 °C for 30 min in PBS/BSA (0.5%) with propidium iodide (0.05 mg/mL) and RNase A (2 µg/mL). The fluorescence was measured using a MACSQuant® Analyzers (Miltenyi, Paris, France). Supplementary Legends to Figures: Figure S1. Human monocytes do not express PRKAA2 mRNA and protein. (A) RT-qPCR analysis of gene expression in monocytes exposed to 100 ng/mL CSF1 (mean ±SD of 3 independent experiments). (B) RT-qPCR analysis of PRKAA2 gene expression in monocytes exposed to 100 ng/mL CSF1 (mean ±SD of 3 independent experiments). The pancreatic PC3 cell line was used as a positive control of PRKAA2 gene expression. (C) Immunoblot analysis of PRKAA2 protein expression in monocytes treated for 2 days with 100 ng/mL CSF1. ACTB was detected as the loading control. 1 Figure S2. Human monocytes do not express STK11 (LKB1) and STK11 knockdown fails to affect monocyte differentiation. (A) RT-qPCR analysis of STK11 gene expression in monocytes exposed to 100 ng/mL CSF1 (mean ±SD of 3 independent experiments). The K562 cell line was used as a positive control of STK11 expression. (B) Monocytes were transfected with siRNAs targeting LUCIFERASE (LUC) or STK11 and exposed for 2 day to 100 ng/mL CSF1. Differentiation was examined as previously described. The percentage indicates the cells which express both TFRC and CD163. (C) K562 cells were transfected with siRNAs targeting LUCIFERASE (LUC) or STK11. The expression of STK11 was analyzed by immunoblotting 48 h after transfection. ACTB was detected as the loading control. Figure S3. Increased P2RY6 expression and decreased P2RY1, P2RY2, P2RY4 and P2RY14 expression in CSF1-stimulated monocytes. (A) RT-qPCR analysis of P2RY gene expression in monocytes exposed to 100 ng/mL CSF1 for the indicated times (mean ±SD of 3 independent experiments). (B) The membrane expression of P2RY6 was measured using flow cytometry after stimulation of human monocytes with 100 ng/mL CSF1 for the indicated times. The results are expressed as the fold induction compared to the unstimulated condition and represent the mean ± SD of 3 independent experiments performed in duplicate. **P <0.01 and ***P <0.001 according to a paired Student t test. Figure S4. The CSF1R receptor colocalizes with the P2RY6 receptor and regulates its expression at the cell surface. Human monocytes were exposed for 1 day to 100 ng/mL CSF1. (A) The expression of P2RY6 and CSF1R was analyzed by immunofluorescence (nuclear staining in blue, P2RY6 in green and CSF1R in red). One representative of 3 independent experiments is shown. (B) The expression of the P2RY6 receptor in transfected monocytes was analyzed by flow cytometry. The results are expressed as the fold induction compared to LUC siRNA and represent the mean ± SD of 3 independent experiments performed in duplicate. 2 Figure S5. P2RY6 but not P2RY1 inhibition reduces CSF1-induced human monocyte differentiation. (A) Human monocytes were exposed for 2 days to 100 ng/mL CSF1 alone or in combination with 5 µM MRS2578 or 5 µM MRS2179, which were added 45 min before CSF1 treatment. Differentiation was assessed as previously described. The percentage indicates the cells that express both TFRC and CD163. (B) The expression of Phospho-PRKAA1, PRKAA1 and LC3B was analyzed by immunoblotting. ACTB was detected as the loading control. (C) Enriched bone marrow monocytes obtained from WT or prkag1-/- mice were exposed for 4 days to 100 ng/mL CSF1. Differentiation was evaluated by a functional assay and the expression of PRKAA1 and LC3 was analyzed by western blot. The results of functional assay are expressed as the number of phagocytosed bacteria per cell and represent the mean ± SD of 3 independent experiments performed in triplicate. ***P<0.001 (vs WT) according to a paired Student t test. The expression of PRKAA1 and LC3 was analyzed by immunoblotting. ACTB detected used as the loading control. (D) Human monocytes were exposed for 2 days to 100 ng/mL CSF1 alone or in association with either 2 µM DRS or 10 µM STO. Where indicated, 15 nM bafilomycin A1 (BafA1) was added 3 h before the end of CSF1 treatment. Protein expression was analyzed by immunoblot. ACTB was detected as the loading control. Figure S6. A high contingent of granulocytic subpopulation blocks PRKAA1 expression and phosphorylation in coculture. Following negative sorting, CD14+ and CD24- (monocytes), as well as CD14- and CD24+ (immature granulocytes) populations from two CMML patients were analyzed separately or together by flow cytometry and western blot. (A) Flow cytometric analysis of CD14 and CD24, identifying CD14 + and CD24-, as well as CD14and CD24+ cells in the two CMML patients. (B and C) CD14+ and CD24- (monocytes), as well as CD14- and CD24+ (immature granulocytes) populations from two CMML patients were cultured separately or together with CSF1 alone or in the presence of 100 µM UDP or 30 µM MRS2693 for the indicated times. The expression of TFRC, phospho-ULK1, ULK1, phospho-PRKAA1, and PRKAA1 was analyzed by immunoblotting. ACTB was detected as a loading control. 3 Figure S7. Pharmacological inhibition of PRKAA1, CAMKK2, PLCs or P2RY6 does not trigger apoptosis in monocytes treated with CSF1. Human monocytes were exposed for 2 days to 100 ng/mL CSF1 alone or in combination with 2 µM DRS, 10 µM STO-609, 1 µM U73122 or 5 µM MRS2578 which were added 45 min before treatment with 100 ng/mL CSF1. (A) Flow cytometry analysis of monocytes stained with ANXA5 (B) Expression of PARP (poly [ADP-ribose] polymerase) was analyzed by immunoblotting. ACTB was detected as the loading control. (C) Monocytes were transfected with siRNAs targeting LUCIFERASE (LUC) or PRKAA1 and exposed 2 days to 100 ng/mL CSF1. The number of cells was determined using a MACSQuant® Analyzer (Miltenyi). The results are expressed as the fold induction compared to LUC siRNA and represent the mean ± SD of 6 independent experiments performed in duplicate. (D) Monocytes were transfected with siRNAs targeting LUCIFERASE (LUC) or PRKAA1 and exposed 2 days to 100 ng/mL CSF1. Cell cycle was performed as described in the supplementary methods section. 4