pip2643-sup-0001-supplementary
advertisement

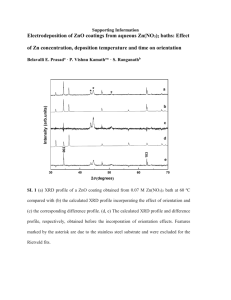
Supporting Information 8.2% Pure Selenide Kesterite Thin Film Solar Cells from Large Area Electrodeposited Precursors Laura Vauche*, Lisa Risch, Yudania Sánchez, Mirjana Dimitrievska, Marcel Pasquinelli, Thomas Goislard de Monsabert, Pierre-Philippe Grand, Salvador Jaime-Ferrer and Edgardo Saucedo Figure S1 shows the evolution of the electrodeposited precursor and the pre-alloyed precursor with ageing. It is evident that the electrodeposited precursor changes while the pre-alloyed precursor is stable over time. Indeed, in the electrodeposited precursor, after a few hours or days, zinc diffuses towards the copper layer to form the Zn-rich alloy CuZn5. After a longer period of time, zinc diffuses deeper into the bulk and forms the more stable Cu5Zn8 alloy. No tin alloy is detected. By contrast, pre-alloying promotes the formation of Cu-Zn and Cu-Sn alloys, which remain unchanged over a time period up to 6 months. No further diffusion of the elements is observed after pre-alloying. Figure S1: XRD and GDOES of the metallic precursor, a) and c) as-electrodeposited; and b) and d) after 200°C annealing. Figure S2 represents SEM images combined with EDX point measurements and mappings. Zn-rich and Sn-rich secondary phases are detected. Higher accelerating voltage (20 kV) – leading to a deeper penetration of the electron beam- was used for the detection of ZnSe secondary phase in order to distinguish the Cu Kα (8.04 keV) and the Zn Kα (8.63 keV) lines. c) Spectrum1 Spectrum2 Acceleration voltage [kV] 15 20 Cu [atom. %] 3.68 10.67 Sn [atom. %] 42.96 8.16 Zn [atom. %] 1.50 32.74 Se [atom. %] 51.86 48.43 Figure S2: a) SEM image and EDX mapping of a Sn-Se grain. The elements are represented by different color (Cu in red, Zn in white, Sn in yellow, Se in green), from black (no presence of the element) to full color scale (presence of the element). b) SEM image of a Zn-Se grain. c) Respective EDX measurements. Figure S3 illustrates the XRD pattern of absorbers with different compositions. The effect of the applied etching can be seen when comparing the intensity of the Sn-Se related XRD peak at around 31°. Figure S3: XRD pattern of the absorber after annealing (black) and after etching (pink for SnSe etching and green for ZnSe+SnSe etching). An intense peak around 31° is attributed to the most intense powder diffraction peaks of SnSe and SnSe2, and is also observed in ref [1-4]. The Sn-Se peak is detected in Sn-rich and in very Cu-poor films. After etching, the Sn-Se peak tends to diminish or disappear, confirming the removal of Sn-Se phases from the surface. [1] S. Temgoua, R. Bodeux, N. Naghavi, S. Delbos, Thin Solid Films 2014, 2. [2] N. Vora, J. Blackburn, I. Repins, C. Beall, B. To, J. Pankow, G. Teeter, M. Young, R. Noufi, J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2012, 30, 051201. [3] M. Ganchev, J. Iljina, L. Kaupmees, T. Raadik, O. Volobujeva, a. Mere, M. Altosaar, J. Raudoja, E. Mellikov, Thin Solid Films 2011, 519, 7394. [4] R. A. Wibowo, S. a. Moeckel, H. Yoo, C. Hetzner, A. Hoelzing, P. Wellmann, R. Hock, Mater. Chem. Phys. 2013, 142, 311.