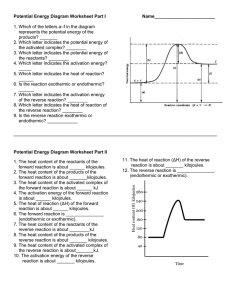
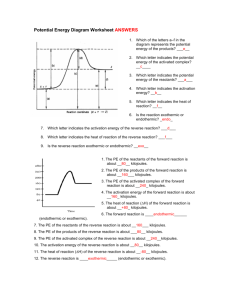
ENERGY DIAGRAM PRACTICE1 Heat of reaction is the amount of
advertisement
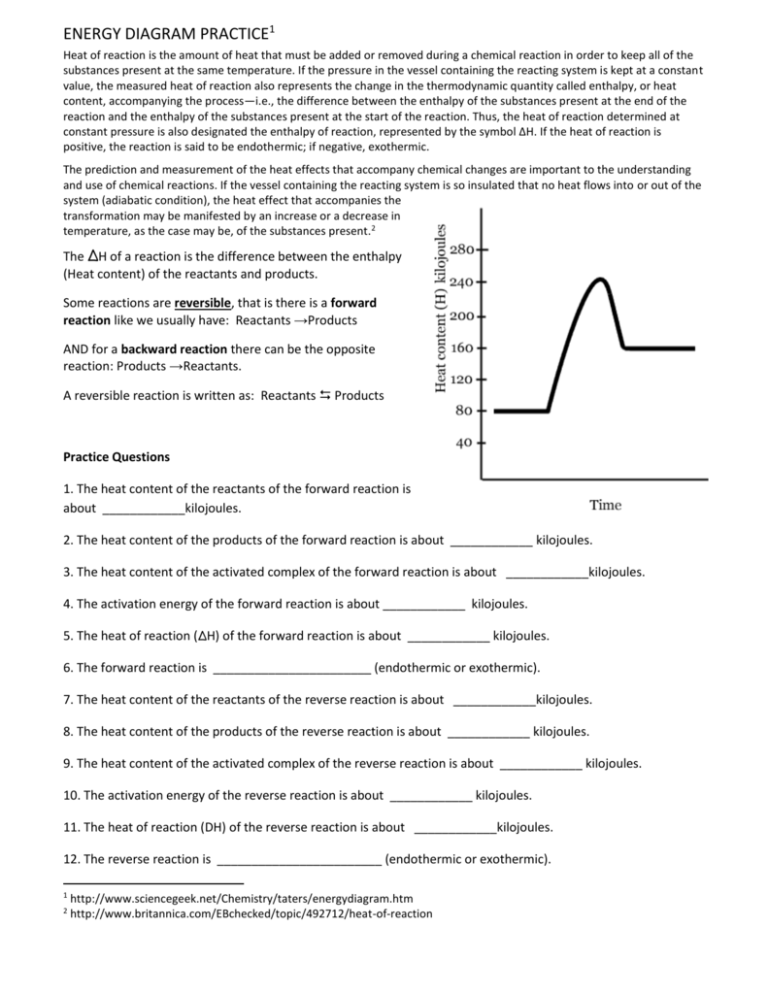
ENERGY DIAGRAM PRACTICE1 Heat of reaction is the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the same temperature. If the pressure in the vessel containing the reacting system is kept at a constant value, the measured heat of reaction also represents the change in the thermodynamic quantity called enthalpy, or heat content, accompanying the process—i.e., the difference between the enthalpy of the substances present at the end of the reaction and the enthalpy of the substances present at the start of the reaction. Thus, the heat of reaction determined at constant pressure is also designated the enthalpy of reaction, represented by the symbol ΔH. If the heat of reaction is positive, the reaction is said to be endothermic; if negative, exothermic. The prediction and measurement of the heat effects that accompany chemical changes are important to the understanding and use of chemical reactions. If the vessel containing the reacting system is so insulated that no heat flows into or out of the system (adiabatic condition), the heat effect that accompanies the transformation may be manifested by an increase or a decrease in temperature, as the case may be, of the substances present.2 The ∆H of a reaction is the difference between the enthalpy (Heat content) of the reactants and products. Some reactions are reversible, that is there is a forward reaction like we usually have: Reactants →Products AND for a backward reaction there can be the opposite reaction: Products →Reactants. A reversible reaction is written as: Reactants Products Practice Questions 1. The heat content of the reactants of the forward reaction is about ____________kilojoules. 2. The heat content of the products of the forward reaction is about ____________ kilojoules. 3. The heat content of the activated complex of the forward reaction is about ____________kilojoules. 4. The activation energy of the forward reaction is about ____________ kilojoules. 5. The heat of reaction (∆H) of the forward reaction is about ____________ kilojoules. 6. The forward reaction is _______________________ (endothermic or exothermic). 7. The heat content of the reactants of the reverse reaction is about ____________kilojoules. 8. The heat content of the products of the reverse reaction is about ____________ kilojoules. 9. The heat content of the activated complex of the reverse reaction is about ____________ kilojoules. 10. The activation energy of the reverse reaction is about ____________ kilojoules. 11. The heat of reaction (DH) of the reverse reaction is about ____________kilojoules. 12. The reverse reaction is ________________________ (endothermic or exothermic). 1 2 http://www.sciencegeek.net/Chemistry/taters/energydiagram.htm http://www.britannica.com/EBchecked/topic/492712/heat-of-reaction