UNIT 3.1 - Revisit NAME: Per._________Date: Atoms, Elements
advertisement

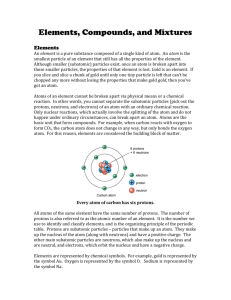
UNIT 3.1 - Revisit NAME:________________________________________ Per._________Date:____________________ Atoms, Elements, Compounds & Mixtures Revisit Part I: Read the following information on atoms, elements, compounds and mixtures. Fill in the blanks where necessary. Atoms: o There are three sub-atomic particles. The subatomic particle with a negative charge is the ________________. The subatomic particle with a ________________________charge is the proton. The subatomic particle that does ______________ have a charge is called the ______________________. o The __________________ of an element is the number of protons and neutrons in the nucleus. o The __________________ ____________ is an average of the masses of all naturally occurring isotopes of an element. o To find the number of ____________________ in the atom, you must subtract the number of protons from the atomic mass. Elements: o A pure substance containing only one kind of ____________. o An element _____________ be separated into simpler materials (except during nuclear reactions). o Over 100 existing elements are listed and classified on the ____________________ ______________. Compounds: o A pure substance containing two or more kinds of _______________. o The atoms are _________________ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. o Compounds ___________________ be separated by physical means. Separating a compound requires a chemical reaction. o The properties of a ___________________are different than the properties of the elements it contains. It forms a new substance with new properties. Mixtures: o Two or more ________________ and/or _________________ NOT chemically combined. o No reaction between substances. o Mixtures can be uniform (called ________________________) and are known as solutions. o Mixtures can also be non-uniform (called ________________________). o Mixtures can be separated into their components by ________________ means. o The properties of a ________________ are similar to the properties of its components. Part II on o Particles in a _______________________ settle out at the bottom of the container. Back…. o Particles in a _______________________ do NOT settle out. NAME:________________________________________ UNIT 3.1 - Revisit Per._________Date:____________________ Part 2: Decide what each of the following items should be labeled as: elements (E), compounds (C) or Mixtures (M). Write the letter X if it is none of these. ___Diamond (C) ___Air ___Krypton (K) ___Water (H2O) ___Ammonia (NH3) ___Wood ___Dry Ice (CO2) ___Sugar (C6H12O6) ___Sulfuric Acid (H2SO4) ___Bismuth (Bi) ___Alcohol (CH3OH) ___Salt (NaCl) ___Bronze ___Baking Soda (NaHCO3) ___Milk ___Gasoline ___Uranium (U) ___Pail of Garbage ___Energy ___Ink ___Titanium (Ti) ___Iron (Fe) ___Electricity ___Popcorn ___A dog ___Gold (Au) ___Pizza ___Concrete Part 3: Match each diagram with its correct description. Diagrams will be used once. A B C D ___1. Pure Element – only one type of atom present. ___2. Mixture of two elements – two types of uncombined atoms present. ___3. Pure compound – only one type of compound present. ___4. Mixture of two compounds – two types of compounds present. ___5. Mixture of a compound and an element. Part 4: Identify by name letters a – d. a)_______________________________ b)_______________________________ c)_______________________________ d)_______________________________ What is the atomic number of this element?_____________________ What is the name and symbol of this element? _______________________________________ E NAME:________________________________________ UNIT 3.1 - Revisit Per._________Date:____________________ Atoms, Elements, Compounds & Mixtures Revisit Part I: Read the following information on atoms, elements, compounds and mixtures. Fill in the blanks where necessary. Atoms: o There are three sub-atomic particles. The subatomic particle with a negative charge is the Electron . The subatomic particle with a Positive subatomic particle that does o The Mass o The Atomic NOT charge is the proton. The have a charge is called the Neutron . of an element is the number of protons and neutrons in the nucleus. Mass is an average of the masses of all naturally occurring isotopes of an element. o To find the number of Neutrons in the atom, you must subtract the number of protons from the atomic mass. Elements: o A pure substance containing only one kind of Atom . o An element can’t be separated into simpler materials (except during nuclear reactions). o Over 100 existing elements are listed and classified on the Periodic Compounds: o A pure substance containing two or more kinds of elements Table . . o The atoms are chemically combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. o Compounds can’t be separated by physical means. Separating a compound requires a chemical reaction. o The properties of a Compound are different than the properties of the elements it contains. It forms a new substance with new properties. Mixtures: o Two or more elements and/or compounds NOT chemically combined. o No reaction between substances. o Mixtures can be uniform (called Homogenous ) and are known as solutions. o Mixtures can also be non-uniform (called Heterogenous ). o Mixtures can be separated into their components by physical o The properties of a o Particles in a o Particles in a means. mixture are similar to the properties of its components. Part II on suspension settle out at the bottom of the container. Back…. colloid do NOT settle out. NAME:________________________________________ UNIT 3.1 - Revisit Per._________Date:____________________ Part 2: Decide what each of the following items should be labeled as: elements (E), compounds (C) or Mixtures (M). Write the letter X if it is none of these. _E_Diamond (C) _M_Air _E_Krypton (K) _C_Water (H2O) _C_Ammonia (NH3) _M_Wood _C_Dry Ice (CO2) _C_Sugar (C6H12O6) _C_Sulfuric Acid (H2SO4) _E_Bismuth (Bi) _C_Alcohol (CH3OH) _C_Salt (NaCl) _M_Bronze _C_Baking Soda (NaHCO3) _M_Milk _M_Gasoline _E_Uranium (U) _M_Pail of Garbage _X_Energy _M_Ink _E_Titanium (Ti) _E_Iron (Fe) _X_Electricity _M_Popcorn _M_A dog _E_Gold (Au) _M_Pizza _M_Concrete Part 3: Match each diagram with its correct description. Diagrams will be used once. A B C D _C_1. Pure Element – only one type of atom present. _E_2. Mixture of two elements – two types of uncombined atoms present. _B_3. Pure compound – only one type of compound present. _A_4. Mixture of two compounds – two types of compounds present. _D_5. Mixture of a compound and an element. Part 4: Identify by name letters a – d. Electron – negative a)_______________________________ Neutron – no charge b)_______________________________ Nucleus – mass of atom c)_______________________________ Proton - positive d)_______________________________ What is the atomic number of this 4 element?_____________________ What is the name and symbol of this element? Beryllium - Be _______________________________________ E