Biomaterials Functionalized with Insulin can enhance
advertisement

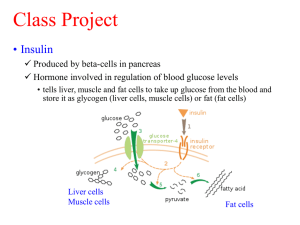
Biomaterials Functionalized with Insulin can enhance Osteoblasts Activity Elena Mavropoulos1, Moema Hausen1, Jéssica Dornelas da Silva1, Suzana Azevedo dos Anjos1-2, Gutemberg Alves2, Alexandre Malta Rossi1 1 Depto. de Física Aplicada, Laboratório de Cultura Celular, CBPF, Rio de Janeiro (RJ), Brasil 2 Depto. de Biologia Celular e Molecular, HUAP, Universidade Federal Fluminense, Niterói (RJ), Brasil. E-mail: elena@cbpf.br Abstract. Hydroxyapatite (HA), zinc substituted hydroxyapatite (ZnHA) and carbonated hydroxyapatite (CHA) are already studied as implanted biomaterial in bone grafts due to its similar chemical composition with bone and due to their biocompatibility, bioresorption and bioactivity properties. HA, ZnHA and CHA powders were synthesized from wet method at 37° C and the DRX patterns showed that the samples presented low crystallinity compared with materials produced at higher temperature. FTIRATR analysis revealed that insulin is present to samples disks after adsorption. Cell attachment assays were performed by fluorescence microscopy and showed a strong increase in attachment in insulin coated samples. The results here obtained showed that insulin coatings on biomaterials could be a promising alternative to rapidly mobilize cells to favor osteointegration. Keywords: Hydroxyapatite, Insulin, surface interaction, osteointegration 1. INTRODUCTION Calcium phosphates are the major mineral constituent of bone and teeth and they are commonly used as a filler of bone defects or as a coating to promote bone formation into orthopedic implants. More recently the focus is to design “smart” biomaterials by modifying the biomaterials surfaces through adsorbing proteins or peptide sequences obtaining a desired surface to control cell response and enhance tissue regeneration [1]. Protein–surface interactions are the major responsible for the biocompatibility of medical devices, since cell settlement to the implanted biomaterial surface, will directly interact with the adsorbed protein layer. According to the surface, a positive approach in the bone cells is expected: the cytoskeleton prompt mobilization and the establishment of new focal contacts. In in vivo models, one negative consequence for delay in the cells adhesion process of bone grafts is unattachment followed by cell death and the formation of a fibrous encapsulation of the material. So on, in vitro models of materials that could generate increased cell attachment are potencial candidates for tests in vivo. Insulin is a polypeptide hormone that acts as a growth-stimulating factor for some cells types in culture, it travels around the bloodstream and is a small, predominantly α-helical protein consisting of 51 residues. The insulin monomer assembles as dimmers (diffused in the blood neutral pH) and in the presence of zinc ions, as hexamers. Some authors [2] showed that bone cells express insulin receptors which are directly correlated to osteoblast proliferation and differentiation. Also it is known that diabetic’s high insulinemia can cause atherosclerosis in patients in a role that is directly linked to an increase in leukocytes adhesion to blood vessels wall [3]. There is a noticeable lack in literature of the insulin interaction in cell adhesion and apparently there is no previous evaluation in any biomaterial. Herein we first report the effects of three different apatite compounds funcionalized with human insulin to interact with osteoblastic cells. A qualitative evaluation of each apatite sample through fluorescence microscopy was performed. Cell attachment analysis of early adhesion was carried out. 2. MATERIALS AND METHODS 2.1 Samples preparation HA and ZnHA powder samples were prepared by dropwise addition of Ca(NO3)2.4H2O and Zn(NO3)2 solution to (NH4)2HPO4 aqueous solution, at 37°C and pH was kept around 9 by addition of KOH. After 2 hours the precipitated was separated by filtration and repeatedly washed with cold water. CHA powder was synthesized from calcium nitrate tetrahydrate, ammonium di-hydrogen phosphate and ammonium carbonate extra pure (Merck) salts dissolved in aqueous solutions. The reagents were mixtured and maintained at 37°C for 2 hours and pH maintained at 13.0 with KOH. All the suspensions were lyophilized and the dried powders were manually grounded, with the < 210 µm particles separated by sieving. For the in vitro assays the powder was uniaxially pressed at 2 Ton to form discs with diameter of 10mm and thickness of 1 mm. The obtained disks were washed three times with ethanol 70% and Milli-Q water and sterilized by gamma radiation. Each apatite disk (HA, ZnHA or CHA) was adsorbed for 24h with 20µg/mL of human insulin (Ins). The insulin-free groups were adsorbed with saline buffer only (PBS). 2.2 Samples Characterization The characterization of the synthesized powders was performed by powder Xray diffraction (XRD) and Fourier-transformed infrared spectroscopy (FTIR). XRD analysis was carried out to determine the crystallinity of the samples and to identify phase composition. The analysis was performed using a SEIFERT-FPM GmbH diffractometer operating with CuKα radiation (1.5418 Å) at 40 kV and 40 mA with a graphite monochromator in the primary bunch. The XRD patterns were obtained in the interval of 2θ step interval from 10 to 100°. Fourier Transformed Infrared (FTIR) was performed using a Shimatzu IR- Prestige-21/AIM-880 operating from 400 to 4000 cm-1. 2.3 Cell culture For the testing of biocompatibility the murine preosteoblast cell line MC3T3-E1 subclone 14 was chosen as these cells are highly differentiated and behave most like osteoblasts. Cells were cultured with α-MEM with 10% fetal bovine serum (FBS) and incubated at 37°C at 5% CO2 atmosphere. Confluent low passages were trypsinized, counted in a Neubauer chamber and used in experiments. The control group of each assay was seeded on Thermanox coverslips (Th) previously coated with 0.1% porcine gelatin (Gel) or 20µg/ml of insulin (Ins). 2.4 Cell attachment and proliferation by fluorescence microscopy The osteoblastic cells were seeded over disks of each material at a density of 0.5x10 cells and incubated at 37ºC/5% CO2. After incubation of 4h, cells were fixed with PFA 4% for 20min, washed in PBS, and permeabilized with Triton 0.1%. After three PBS washes, each disk was immersed in 4% albumin for 30min. Cells were stained for fluorescence with DAPI 1:1000 for 15min and phalloidin-TRITC 1:100 for 1h at RT. Disks were immersed on antifading solution, mounted on coverslips and observed in an inverted fluorescence microscope (AxioObserverA1, Zeiss, Germany). The cell quantification was performed by Image Pro Plus 6.0 software (Media Cybernetics Inc., USA). 4 3. RESULTS AND DISCUSSION 3.1 HA, ZnHA and CHA samples characterization The peaks position and peaks linewidths for all powder samples corresponded to a well-crystallized hydroxyapatite (JCPDS 09-0432), as shown in Fig. 1. Comparing the XRD patterns of HA, CHA and ZnHA powder produced at 37°C no significant changes in respect to the crystallinity were observed. The HA and CHA samples presented the main characteristics peaks for hydroxyapatite phase according to data Powder Diffraction File 89-4405 JCPDS (211, 112 and 300 planes) indexed as shown in figure 1. Broader peaks for ZnHA sample causing the overlap of 211, 112 and 300 planes. Figure 1. XRD pattern of CHA 37°C, HA 37°C and ZnHA 37°C. Fourier Transformed Infrared Attenuated Total Reflectance Microscopy (FTIRM-ATR) spectroscopy is a suitable tool to detect protein conformational changes after adsorption experiments [4]. The FTIRM-ATR analysis of HA, ZnHA and CHA disks before and after insulin adsorption showed additional bands after insulin adsorption that were attributed to protein presence (data not shown). 3.2 Cell attachment by fluorescence microscopy The insulin adsorbed materials in all samples increased the cell spreading after 4h of incubation (Figure 2). The total cell count by nuclei quantification was the same in all samples after 4h (data not shown). The stress fibers were observed mainly by Th + Ins and CHA + Ins groups. A striking difference in the cell spreading by the thermanox (Th) with and without insulin was evident. Comparing the morphological presentation of the three analyzed ceramics, the ZnHA + Ins group showed the lower cell spreading while CHA + Ins showed the higher spreading. Is well known that integrins are directly related to cell adhesion. According to some authors [5] the integrins play a role in bone formation. These authors showed that when cell were exposed to insulin-like growth factor 1 (IGF-1), the osteoblast cells increased activity and changed morphology. Others authors [6] also showed that IGF-1 stimulated osteoblast adhesion. According to these authors, several matrix proteins in bone contain the RGD sequence and may be involved in the binding of the osteoblast to osteoid through integrins, so on when cells were treated with IGF-1 they became flatted and increased the binding to many matrix proteins. It´s possible that the increase in cell spreading observed here in insulin coatings rapidly increased the integrin expression outcoming in higher adhesion. The insulin molecule has no binding site to the osteoblastic cell integrin although the cells posses insulin receptors. Thus, the increased adhesion here observed is probably not related to a direct adhesion to the insulin itself, but due to an increase in the recruitment of integrin expression, outcoming the higher adhesion to the materials. The insulin may have acted as an IGF-1 as previously reported by these authors. Regarding the concept of smart materials to induce a rapid accession to favor osseointegration, the coating materials with insulin seems to be a promising alternative to bone grafts. Futher analysis of specific bone signaling molecules are to be investigated in order to full understand the role of insulin in bone cells adhesion, proliferation and differentiation. Th + Gelatin Th + Ins HA + PBS HA + Ins ZnHA + PBS ZnHA + Ins CHA + PBS CHA + Ins Figure 2. The cell adhesion after 4h in each material adsorbed with insulin (right column) and with PBS (left column) showed that cell spreading is highly increased when in the presence of insulin. Typical stress fibers usually observed in late adhesion times were observed in Th + Ins and the general polygonal morphology of this lineage was also observed. 4. ACKNOWLEDGMENTS The authors would like to thank CNPq, CAPES and FAPERJ for financial support and Luciana Consentino for technical support in cell culture. 5. REFERENCES [1] Song J. A chemical and engineering approach towards "smart" synthetic bone grafts. J Musculoskelet Neuronal Interact. 2007 Oct-Dec;7(4):325 [2] Mathieu Ferron, Jianwen Wei, Tatsuya Yoshizawa, Andrea Del Fattore, Ronald A. DePinho, Anna Teti, Patricia Ducy, Gerard Karsenty. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism. Cell 142, 296–308, July 23, 2010 Elsevier Inc. [3] Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2010 [4] Mavropoulos E, Costa AM, Costa LT, Achete CA, Mello A, Granjeiro JM, Rossi AM Adsorption and bioactivity studies of albumin onto hydroxyapatite surface. Colloids Surf B Biointerfaces. 2011 Mar;83(1):1-9 [5] Gohel AR, Hand AR, Gronowicz GA. Immunogold localization of beta 1-integrin in bone: effect of glucocorticoids and insulin-like growth factor I on integrins and osteocyte formation. J Histochem Cytochem. 1995 Nov;43(11):1085-96 [6] Gronowicz G, McCarthy MB, hegot A. IGF-1 increases integrin levels and osteoblast adhesion. J Bone Miner Res. 1993;8:S308