tatatalolol
advertisement

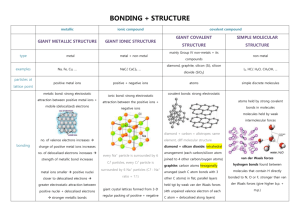
2. b. Students know chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. Organic and biological molecules usually are made of H2, Ch4, NH3, H2CCH2, N2, and Cl2. These elements all share valance electrons and this is how they form bonds. The outer energy levels of the electron configurations are filled in combination with atoms in molecules of other combinations. Such as Carbon uses its four unpaired electrons and forms a combination with hydrogen, nitrogen, and oxygen to form covalent bonds sharing electron pairs. The usage of the covalent bond process helps the different molecules form different combinations. 2. c. Students know salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction. Electrostatic attraction is the attraction between anions, negative ions, and catitons, positive ions. The energy that is created is called Lattice Energy, and it is what holds ionic compounds together. The salt crystals utilize this process to create structures that form repeating patterns to create less distance between positive and negative ions. 2. d. Students know the atoms and molecules in liquids move in a random pattern relative to one another because the intermolecular forces are too weak to hold the atoms or molecules in a solid form. In a liquid, there is no basic structure but that of the container holding it. The molecules are all touching, but they are free to move away from the rest of the molecules if there is no barrier. This is because in a liquid, the intermolecular forces that are holding the particles together are really weak. This force is often overcome by the force of momentum and causes the molecular formation to lose shape, thus allowing the moving of the particles in the liquid form. 2. e. Students know how to draw Lewis dot structures. A Lewis dot structure is a type of diagram that shows the way that valance electrons and covalent bonds are arranged between atoms in a molecule. The periodic table is used to determing the amount of valance electrons that a certain element has. The elements that are being combined are then drawn out with the corresponding amount of valance electrons and they are arranged in a manner in which all elements have their maximum amount of valence electrons. 2. f. Students know how to predict the shape of simple molecules and their polarity from Lewis dot structures. Lewis dot structures give a glimpse of the molecular structure of the atoms in a molecule. It also shows how electrons are being shared between atoms with a line, the extra molecules that are not used are represented by a dot. \ Lewis Dot Structure for TeCl4