Percent Composition and Empircal Formula - Notes

Name_______________________________________ Formula Calculation – NOTES Date__________ Period______
Dalton’s atomic theory
1.________________________________________________________________________________________________
2.________________________________________________________________________________________________
________________________________________________________________________________________________
3.________________________________________________________________________________________________
4.________________________________________________________________________________________________
________________________________________________________________________________________________
5.________________________________________________________________________________________________
Definitions: (p.68)
Law of Conservation of mass:__________________________________________________________________________
__________________________________________________________________________________________________
Law of Definite Proportions:___________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
Law of Multiple Proportions:___________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
Molar Mass - Mass of 1 mole of an element or compound.
Atomic mass tells the...
atomic mass units per atom (amu)
grams per mole (g/mol)
Ex1 – Find the molar mass of Cu
2
S.
P1 – Find the molar mass of Cr
2
(SO
4
)
3
What is the name of Cu
2
S?______________________________
What is its name?___________________________
Percentage Composition - the percentage by mass of each element in a compound
Ex2 - Find the % composition of Cu
2
S.
- determine the molar mass
- divide the mass of each element by the molar mass
- multiple by 100
P2 – Find the % composition of Cr
2
(SO
4
)
3
Ex3 - Find the percentage composition of a sample that is
28 g Fe and 8.0 g O.
- determine the mass of the sample
- divide the mass of each by the total sample mass
- multiple by 100
P3 – Find the % composition of a sample that is 53.04 g
Cr, 49.06 g S, and 97.9 g O.
Ex4 - How many grams of copper are in a 38.0-gram sample
of Cu
2
S?
- determine the molar mass
- calculate the % composition of copper
- multiple mass of sample by % of copper
Ex5 - Find the mass percentage of water in calcium
chloride dihydrate, CaCl
2
•2H
2
O?
- determine the molar mass of water and the entire compound
- divide the molar mass of water by the molar mass of compound
- multiple by 100
P4 – How many grams of Cr are in a 31.5 g sample of
Cr
2
(SO
4
)
3
?
P5 – Find the mass % of water in Cr
2
(SO
4
)
3 °
10H
2
O.
Empirical Formula - Smallest whole number ratio of atoms in a compound
General guidelines for determining empirical formula
1. Determine # of moles of each element in the formula.
- if given mass of element, simple divide by molar mass of element to get # of moles.
- if given % of each element, assume you are dealing with a 100g sample.
2. Divide moles by the smallest # to find subscripts.
3. When necessary, multiply subscripts by 2, 3, or 4 to get whole #’s.
P6 – Find the empirical formula for a sample that Ex6 - Find the empirical formula for a sample that contains
297.2 g of Fe and 127.8 g of O.
- convert to moles
- divide moles by the smallest # to find subscripts.
contains 2.67 g of carbon and 7.11 g of oxygen.
Ex7 - Find the empirical formula for a sample of 25.9% N and
74.1% O.
- convert the % into masses
- convert to moles
- divide moles by the smallest # to find subscripts.
P7 – Determine the Empirical formula for the 400 gram sample containing 31.5% cobalt, 34.28% sulfur, and
34.22% oxygen.
Molecular Formula - “True Formula” - the actual number of atoms in a compound
Determining Molecular Formula
1. Find the empirical formula.
2. Find the empirical formula mass.
3. Divide the molecular mass by the empirical mass.
4. Multiply each subscript by the answer from step 3.
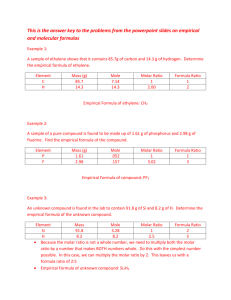
Ex8 - The empirical formula for ethylene is CH
2
. Find the
molecular formula if the molecular mass is 28.1 g/mol?
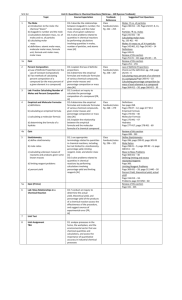
P7 – The empirical formula for a common drying agent is P
2
O
5
. The molecule has a molar mass of 283.88 g/mol. Find the molecular formula of the compound.