This is the answer key to the problems from the powerpoint slides on
advertisement
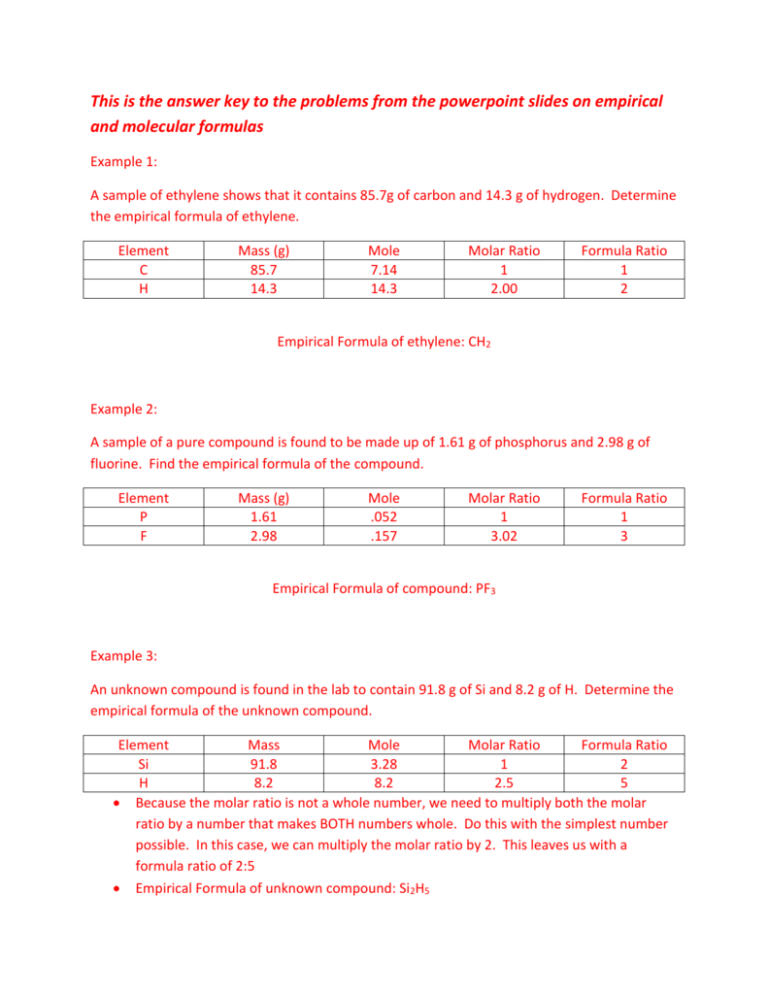
This is the answer key to the problems from the powerpoint slides on empirical and molecular formulas Example 1: A sample of ethylene shows that it contains 85.7g of carbon and 14.3 g of hydrogen. Determine the empirical formula of ethylene. Element C H Mass (g) 85.7 14.3 Mole 7.14 14.3 Molar Ratio 1 2.00 Formula Ratio 1 2 Empirical Formula of ethylene: CH2 Example 2: A sample of a pure compound is found to be made up of 1.61 g of phosphorus and 2.98 g of fluorine. Find the empirical formula of the compound. Element P F Mass (g) 1.61 2.98 Mole .052 .157 Molar Ratio 1 3.02 Formula Ratio 1 3 Empirical Formula of compound: PF3 Example 3: An unknown compound is found in the lab to contain 91.8 g of Si and 8.2 g of H. Determine the empirical formula of the unknown compound. Element Mass Mole Molar Ratio Formula Ratio Si 91.8 3.28 1 2 H 8.2 8.2 2.5 5 Because the molar ratio is not a whole number, we need to multiply both the molar ratio by a number that makes BOTH numbers whole. Do this with the simplest number possible. In this case, we can multiply the molar ratio by 2. This leaves us with a formula ratio of 2:5 Empirical Formula of unknown compound: Si2H5 Example 3 continued: Another experiment indicates that the molar mass of the unknown compound is 122 g/mol. Determine the molecular formula of the compound. In order to determine the molecular formula, you must do the following: 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑒𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 𝑓𝑜𝑟𝑚𝑢𝑙𝑎 Therefore, 122 𝑔/𝑚𝑜𝑙 61 𝑔/𝑚𝑜𝑙 =2 This number “2” tells us how many empirical formula units can go into the molecular formula. Therefore, multiply all the subscripts in the empirical formula by 2 in order to get the molecular formula. (Si2H5) x 2 = Si4H10 = molecular formula of the unknown compound Example 4: A compound contains 52.2% C, 13.0% H, and 34.8% O. Calculate the empirical formula of the compound. If we assume, which we can, that the total mass of the compound is 100 g, then it makes sense that we are able to make the claim that, in this 100g sample, there are 52.2 g C, 13 g H, and 34.8 g O. We will use these values for our masses. Element Mass Mole Molar Ratio Formula Ratio C 52.2 4.35 1.99 2 H 13 13 5.96 6 O 34.8 2.18 1 1 When presented with 3 or more elements, the molar ratio is determined by dividing all the moles of each element by the smallest amount of moles (in this case, oxygen since it only contains 2.18 moles). Therefore, the molar ratio is all RELATIVE to oxygen since it is the least amount. Empirical Formula of compound: C2H6O The empirical formula cannot be reduced any further. You must remember that there is technically a subscript of “1” after the oxygen and that “1” cannot be reduced any further.











