Temperature Controlled Rooms - University Wiki
advertisement

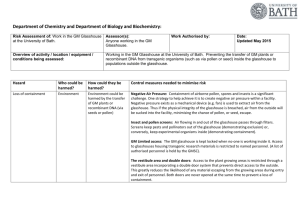
Department of Chemistry and Department of Biology and Biochemistry: Risk Assessment of: Work in the Temperature Controlled Rooms at the University of Bath. Assessor(s): Anyone working in the Temperature Controlled Rooms Work Authorised by: Date: Updated in May 2015 Overview of activity / location / equipment / conditions being assessed: Working in the Temperature Controlled Room at the University of Bath. Preventing the transfer of GM plants or recombinant DNA from transgenic organisms (such as via pollen or seed) inside the temperature controlled rooms to outside. Hazard Who could be harmed? How could they be harmed? Control measures needed to minimise risk Loss of containment Environment Environment could be harmed by the transfer of GM plants or recombinant DNA (via seeds or pollen) GM Limited access: Access to the GM temperature controlled rooms housing transgenic research materials is restricted to personnel who have received GM training and been registered with the GM Committee. Sticky mats: A sticky mat (TakMat) is placed inside the outer doorway of the temperature controlled room deigned to trap any material such as pollen, seeds, etc adhering to foot-ware. The topmost layer of the mat needs regular removal and disposal as contaminated waste. Protective clothing: Within the temperature controlled rooms are designated lab coats. These must be worn at all times by staff in the facility. These should be taken off and left in the vestibule before leaving the facility. This will minimize the chance of spread of GM material. Labelling: All GM plant material is labelled. Where known, end dates are recorded on plant trays and also on the external doors of the temperature controlled rooms. Cleanliness: All areas of the temperature controlled rooms should be cleaned regularly and maintained in a tidy condition. Hygiene facilities such as a washbasin are available and must be made use of by users of the facility. All re-usable materials such as pots, trays etc. should be disinfected by a validated method after each use. When seed collection is taking place, increased hovering should take place. Hoover bags should be regularly changed and autoclaved before disposal. Deep cleaning should take place at the end of experimental work. Collection and treatment of drainage water: All liquid effluent is collected and disinfected in a sump prior to discharge to the drains. Secure transport of plants and materials between Temperature Controlled Rooms, GM Glasshouse and Laboratory areas: Plants and materials containing transgenes should be transported in double sealed containers with lids that minimize the risk of escape. Waste materials: All waste plant material, growth media, floor sweepings, TakMat layers and disposable materials should be segregated into clearly marked bags and treated by a validated means before disposal to kill any residual organisms and destroy any rDNA. Where possible, autoclaving should be used. Minimizing the amount of pollen and seed: Pollen and seed represent the most significant risk to the environment from transgenic plants. Minimize this risk by preventing flowering or seed set where possible; otherwise keep the number of flowers or seed pods to the minimum consistent with the demands of the experiment. Seed storage: Seeds should be securely stored in laboratories, temperature controlled rooms or in the GM glasshouse vestibule fridge. Residual risk rating: (3) Moderate x (2) Unlikely = (6) LOW Untrained staff in facility Environment Environment could be harmed by the transfer of GM plants or recombinant DNA (via seeds or pollen) Providing information, training and supervision: Before entering temperature controlled rooms, all staff working around transgenic organisms should be fully informed about the containment measures applicable to a given research project. Prescribed procedures and practices should be appropriate for the assigned containment level. Relevant GM project risk assessments: The relevant GM project RA must be read and signed by anyone working on GM projects. Appropriate supervision of workers must be provided. Induction material: No staff are allowed to start work until they have been given an induction. The induction includes instruction to all staff to register all GMO work with the GM committee, that they cannot use the temperature controlled facilities without receiving training first and that all work must be Risk Assessed. Staff are regularly sent out an induction summary to remind them of the induction details and to send any new staff to the Technical Team. Disposal: Re-usable pots must be disinfected by immersing in a solution of bleach (1,000ppm available chlorine) for at least 8 hours (usually overnight). Note: 1,000ppm Cl solution can be made by diluting commercial bleach [nominal 5% Cl] by a factor of 1 in 50. All plant material, compost, floor sweepings, TakMat layers and disposable plastic ware, such as pots, should be placed in the autoclave bags provided in the blue skips. N These bags (and skips) will be removed periodically for sterilization by autoclaving (minimum of 126oC for 30 minutes). The floor (including under the floor drain covers) should be cleaned with a vacuum cleaner every week. Material from the vacuum cleaner bag must be disposed of in the autoclave bag. Residual risk rating: (3) Moderate x (2) Unlikely = (6) LOW Unauthorised access to facility Environment Environment could be harmed by the transfer of GM plants or recombinant DNA (via seeds or pollen) Signs are on door inducting that access is restricted. Access to temperature controlled rooms housing transgenic research materials is restricted. Only personnel approved by the GMSC should enter the Temperature Controlled Rooms. A list of approved personnel is available from the GMSC. Visitors should be accompanied by an approved person who should explain the operation of the facility. The inner door should be closed when workers are in the temperature controlled rooms. Residual risk rating: (3) Moderate x (2) Unlikely = (6) LOW GM plants/ plant material on Temperature Controlled Room users Temperature controlled room users Consumption by humans/ contact with humans. Slips and Trips Temperature controlled room users Slipping on floors/ tripping on hazards Chemicals Temperature controlled room users Hazardous chemicals affecting human health All work must be risk assessed and the nature of the plant species being used will be detailed in this. All users must read, understand and sign a Risk Assessment for their work before starting. Any risks to human health will be noted in this Risk Assessment and the methods put in place to reduce risk will be noted and followed. Floors are regularly cleaned with algal-biocide to prevent them becoming slippery. Good house-keeping means that clutter is removed. A glasshouse technician is employed to ensure best practice. They provide training on the use of temperature controlled rooms. All hazardous chemicals to be stored in a chemicals cabinet. Instructions on cleaning products containing chemicals should be followed. Electrical Temperature controlled room users Electrical shock Biological Temperature controlled room users Temperature controlled room users Temperature controlled room users Possible infection. Manual Handling Environment Injury to temperature controlled users. Temperature in hot weather may cause discomfort to users. All temperature controlled room electrical equipment should be PAT tested. Care should be taken to keep electrical items away from any water sources. Do a visual inspection before use – if any damage or defects are seen – do not use until rectified. Ensure good housekeeping and good hygiene to decrease the risk of microorganisms on the skin. No eating or drinking is allowed in any of the temperature controlled rooms. Handwashing facilities are available. The University Manual Handling guidelines should be followed. Users should make sure to take regular breaks outside in the fresh air and keep hydrated. Risk Assessment Guidance The assessor can assign values for the hazard severity (a) and likelihood of occurrence (b) (taking into account the frequency and duration of exposure) on a scale of 1 to 5, then multiply them together to give the rating band: Hazard Severity (a) Likelihood of Occurrence (b) 1 – Remote 2 – Unlikely 3 – Possible 4 – Likely 5 – Very likely (almost never) (occurs rarely) (could occur, but uncommon) (recurrent but not frequent) (occurs frequently) The risk rating (high, medium or low) indicates the level of response required to be taken when designing the action plan. Fatal Serious Moderate (eg discomfort, slight bruising, self-help recovery) (eg small cut, abrasion, basic first aid need) (eg strain, sprain, incapacitation > 3 days) (eg fracture, hospitalisation >24 hrs, incapacitation >4 weeks) (single or multiple) Minor Trivial 1 – Trivial 2 – Minor 3 – Moderate 4 – Serious 5 – Fatal Rating Bands (a x b) Remote 1 2 3 4 5 2 4 6 8 10 3 6 9 12 15 Likely 4 8 12 16 20 Very likely 5 10 15 20 25 Unlikely Possible LOW RISK (1 – 8) MEDIUM RISK (9 - 12) HIGH RISK (15 - 25) Continue, but review periodically to ensure controls remain effective Continue, but implement additional reasonably practicable controls where possible and monitor regularly -STOP THE ACTIVITYIdentify new controls. Activity must not proceed until risks are reduced to a low or medium level