SUPPLEMENTARY MATERIALS 1 PY-GC/MS The pyrolysis of the
advertisement

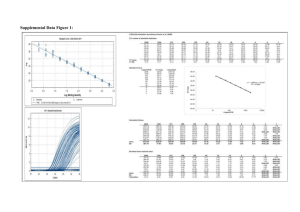
SUPPLEMENTARY MATERIALS 1 PY-GC/MS The pyrolysis of the samples analyzed confirms the presence of the organic materials already identified by GC/MS and summarized in Table S 1. Table S 1 Summary of results from the Py-GC/MS analysis Samples Animal glue Pyrrole PFX 8 PR PFX 15 SX 5 B SX 5 M SX 8 PL SX 10 B SX 10 M SD 2 X X X X X X X X Egg Markers Hexadecanonitrile Octedecnonitrile X X X X - Saccharide materials Levoglucosan Xylofuranose X X X X X 2 GC/MS The quantitative determination of amino acids, aldoses and uronic acids, aliphatic mono- and dicarboxylic acids is performed by using standard solutions, building calibration curves, and evaluating daily recoveries. Running blanks of the procedure highlighted a low level of contamination. The detection limit (LOD) and the quantitation limit (LOQ) of amino acids, aldoses, uronic acids, and fatty and dicarboxylic acids were calculated. At a statistical significance level of 0.05, the LODs and LOQs obtained of the proteinaceous, glycerolipids and saccharide materials were as follows: Proteinaceous materials: LOD: 0.2 μg; LOQ: 0.3 μg Glycerolipids: LOD: 0.3 μg; LOQ: 0.7 μg Saccharide materials: LOD: 0.3 μg; LOQ: 0.5 μg. 2.1 Proteinaceous fraction The proteinaceous content of all the samples analyzed resulted to be higher than LOQ (0.3 µg) and Table S 2 summarizes their amino acidic percentages. Table S 2 Relative percentage of amino acid content and total amount of proteins in the analyzed samples. Ala: alanine, Gly: glycine, Val: valine, Leu: leucine, Ile: isoleucine, Ser: serine, Pro: proline, Phe: phenylalanine, Asp: aspartic acid, Glu: glutamic acid, Hyp: hydroxyproline Amminoacidic Sample Ala Gly Val Leu Ile Ser Pro Phe Asp Glu Hyp YX 1 I 9.9 14.9 9.1 13.6 6.4 4.0 5.9 5.5 13.5 17.4 0.0 0.3 YX 1 PR 9.9 18.3 4.6 6.7 3.2 4.6 11.5 4.4 9.9 17.0 9.7 9138.1 PFX 2 8.8 10.8 8.9 11.4 6.3 4.7 6.8 5.3 20.1 17.0 0.0 0.3 PFX 8 T 10.4 29.8 3.3 6.7 2.9 2.5 8.2 3.5 11.6 14.4 6.6 1.2 PFX 11 T 17.5 44.2 4.1 4.9 2.7 1.5 13.8 1.5 4.7 4.8 0.3 1.8 PFX 11 PL 13.3 33.4 3.5 4.8 2.4 2.3 13.2 2.6 8.9 14.0 1.7 1.4 SX 4 7.9 17.7 3.9 8.5 4.0 8.0 10.0 5.2 19.7 14.2 0.9 0.6 SX 5 T 8.9 25,7 2.7 4.4 1.9 4.8 12.1 3.3 10.0 21.0 5.1 14.4 SX 6 4.9 9.70 1.8 3.6 1.5 9.4 3.0 3.7 16.7 22.7 23.0 10.4 SX 8 T 1.0 39.1 5.9 9.9 4.5 4.0 16.6 2.9 6.5 5.6 4.0 2.1 SX 9 B 3.0 31.3 2.9 4.2 2.1 5.7 8.5 3.1 12.2 18.9 8.2 4.4 SX 9 S 14.7 30.5 8.7 9.8 5.1 5.2 14.5 1.4 31.0 2.8 4.1 0.7 SX 10 M 9.0 23.5 5.5 9.4 4.5 3.3 12.8 5.7 14.3 11.4 0.8 0.5 SX 10 B 8.4 17.3 30.8 9.0 4.1 2.6 7.1 4.1 7.7 7.1 1.9 1.0 SD 3 2.0 25.2 6.4 9.3 5.3 2.6 18.4 4.3 9.2 14.4 2.6 0.7 SD 4 7.2 15.6 20.2 4.5 2.2 4.6 7.8 2.6 7.1 15.2 4.0 16.5 content (µg) Figure S1 shoes the loading plot of the reference samples used as database for the PCA analysis of the amminoacidic profiles obtained from the samples. The loading plot highlights the important role that each amino acid plays in the separation of clusters. Figure S1 Loading plot of the reference samples 2.2 Saccharide fraction Analyses results show that 3 samples present a content of saccharide material higher than LOQ (0.5 µg) while sugars content of 4 samples is between the LOD (0.3 µg) and LOQ. Table S 3 summarizes the glycosidic profiles of the seven samples whose saccharide material is higher than the LOQ and the presence/absence of the singular sugars in those samples showing a saccharide content between the LOD and LOQ. Table S 3 Relative percentage of monosaccharides and uronic acid content, total amount of saccharides in the analyzed samples. Xyl: xylose, Ara: arabinose, Ram: rhamnose, Fuc: fucose, Gal ac: galacturonic acid, Glu ac: glucuronic acid, Glu: glucose, Man: mannose, Gal: galactose Saccahride Sample Xyl Ara Ramn Fuc Galact ac Glu ac Glu Man Galact YX 1 I 18.5 26.4 0.6 0.0 0.0 0.0 26.8 7.8 19.9 44.3 PFX 2 20.7 14.9 1.4 0.7 0.0 0.0 45.8 9.3 7.2 0.9 SX 4 14.3 10.8 2.1 1.0 0.0 2.7 33.4 17.2 18.5 0.5 SX 5 T X X X X - X X X X 0.4 SX 9 B X X - - - - X X X 0.4 SD 3 X X X X - - X X X 0.3 SD 4 X X X X - - X X X 0.3 X= presence of sugar content (µg) 2.3 Lipid fraction Lipid chromatographic profiles reveal the absence of the markers of terpenic resins, shellac and natural waxes. In quite all samples analyzed, the amount of lipid material is generally low although above LOQ (0.7 µg). Table S 4 Relative percentage of lipid, total amount of lipid and their characteristic ratios in the analyzed sample. Lau = lauric acid, Sub = suberic acid, Aze = azelaic acid, Mir = myristic acid, Seb = sebacic acid, Palm = palmitic acid, Oleic = oleic acid, Stear = stearic acid. A/P: azelaic acid over palmitic acid ratio, P/S: palmitic acid over stearic acid ratio, O/S oleic acid over stearic acid ratio, ΣD: sum of dicarboxylic acids Sample Lau Sub Aze Mir Seb Palm Oleic Stear YX 1 I 3.0 0.1 1.4 2.8 0.4 30.2 27.3 34.0 YX 1 PR 2.9 0.5 0.8 2.2 0.2 26.9 32.2 Lipid A/P P/S O/S %ΣD 3.7 0.0 0.9 0.8 2.6 34.3 1.9 0.0 0.8 0.9 1.6 content (µg) 0.7 1.1 2.7 0.3 29.3 34.5 28.3 0.0 1.0 1.2 2.0 PFX 8 T 3.1 1.1 5.7 5.1 0.5 0.9 2.9 0.5 30.6 11.8 0.0 0.6 0.3 1.9 PFX 11 T 12.4 1.4 15.2 0.8 0.0 23.3 4.0 43.0 47.7 3.5 0.5 0.5 0.1 16.5 PFX 11 PL 20.1 0.4 1.6 1.4 0.3 23.7 17.6 34.9 1.2 0.1 0.7 0.5 2.3 SX 4 12.4 3.4 3.1 4.5 1.4 29.8 18.2 37.2 1.2 0.1 0.8 0.2 7.9 PF X 2 SX 5 T 1.1 1.1 2.9 3.8 6.0 37.6 0.8 46.6 4.4 0.1 0.8 0.0 10.1 SX 8 T 16.0 0.0 2.0 3.8 0.3 32.1 10.9 34.9 1.3 0.1 0.9 0.3 2.3 SX 10 M 1.8 4.5 7.3 3.3 1.2 38.4 1.5 42.0 0.6 0.2 0.9 0.0 13.0 SX 10 B 1.0 10.0 36.7 1.8 9.9 18.9 1.8 19.9 1.4 1.9 1.0 0.1 56.6 62.5 1.0 0.0 0.5 0.1 1.2 SD 4 2.5 0.5 0.5 1.2 0.1 29.5 3.1 3 Gilding inorganic materials: combined use of SR micro FT-IR and SR micro XRF Micro XRF results highlighted the compositional difference between the adhesive layer (layer II), characterized by the presence of Mn, Pb, Ca and P, and the white ground layer (layer IV), containing Si, Al, K, Ca and Ti. The detection of Si, Al and K in the white ground layer is in agreement with the presence of a clay identified as kaolinite thanks to the octahedral coordination external O-H absorption peaks (3697, 3669 and 3654 cm-1) in the FTIR spectrum (Fig. 6 section 3.3.). The distribution of Fe, in an area between the adhesive layer and the preparation layer, is in agreement with the red layer that could be observed in the cross-section (Fig. 2 i) and particularly highlighted under UV light (Fig. S 2 c), The presence of Fe and Mn in the red and adhesive layers may point to the use of iron based pigments (Fe2O3 or FeOOH) and MnO2, in agreement with the use of a natural earth named umber [34] as red-orange pigments added to the organic adhesive of the gold foil. In previous work on the mural paintings in the Temples under Chieftain Lu in Liancheng (Gansu Province, China) an adhesive layer made of a binder admixed with red iron oxide was also found under the gold leaf [33]. In the literature, iron based pigments, red lead or other red pigments were commonly used in gilding samples aiming at enhancing the color of gold [19]. Pb was identified into the adhesive layer. The presence of the OH absorption band around 3538 cm-1 (Fig. 6 section 3.3.) combined with strong absorption of COO- asymmetric stretching vibration around 1400 cm-1 supports the identification of hydrocerussite (Pb3(CO3)2(OH)2). It has been previously established that lead white was used either as the major constituent or mixed with other colored pigments to produce different hues in the mural paintings of the Five Northern Provinces’ Assembly Hall [19]. The presence of the characteristic OH stretching bands at 3544 and 3404 cm-1, as well as the OH bending band at 1620 cm-1, is in agreement with the presence of gypsum (CaSO4·2H2O) locally present in the adhesive layer. The interpretation of the presence of phosphorous (P) in layer II and III from the XRF maps is not straightforward. P has been traditionally linked to the presence of bone black (hydroxylapatite), resulting from burning animal bones [19]. However, we cannot confirm its presence by any other means. The use of animal glue as binder, extracted from boiling animal bones, could be a possible explanation to the P distribution though this hypothesis would need further investigation to be confirmed. Fig. S2 (a) SR μXRF maps at 7.2 keV Mn, Ag, Ca, Au, Cl, Al, Fe, K, Pb, P, Si and S acquired for the entire sample with 1×1 μm2 pixel size; (b) scheme of the sample build-up; (c) UV image of the cross-section of sample SD 2