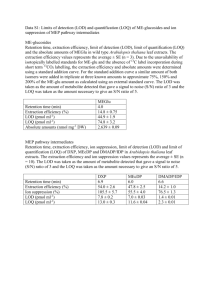
Determination of halogenated volatile organic hydrocarbons in
advertisement

Supporting Information Composition of the surfactant Aerosol OT and its influence on the properties of an agrochemical formulation Johannes Glaubitz1,2, Karl Molt, Torsten C. Schmidt* 1 1 University Duisburg-Essen. Instrumental Analytical Chemistry. Universitätsstr. 5. 45141 Essen. Germany 2 Bayer CropScience, BCS-D-FT-A&S, Alfred-Nobel-Straße 50, 40789 Monheim am Rhein * Corresponding author: E-mail: torsten.schmidt@uni-due.de Phone: +49 201 183-6774 Fax: +49 201 183-6773 Table of Contents 1. Sample for testing on mass calibration of ToF-MS ................................................................................... 4 2. Content of diester 1, monoester 2 and monoester 3 in different production batches of commercially available AOT of different suppliers ................................................................................................................. 6 3. Sedimentation in trail storage formulation samples ............................................................................... 8 4. Centrifugation of a model agrochemical formulation containing AOT of supplier A1 .............................. 8 5. Results of the analysis of AOT of different production batches for inorganic anions and cations of different suppliers ............................................................................................................................................ 9 6. Analysis of the composition of the solvent in AOT on differences between the suppliers ..................... 16 7. Statistical evaluation of the usefulness of the contents of diester 1 and monoester 2 and 3 for product identification .................................................................................................................................................. 21 1 List of Figures Figure S 1: Test on sedimentation after 0.5 a storage at room temperature of a model agrochemical formulation containing AOT of supplier A1, B and D. Increasing amount of visible sediment from supplier A1 to supplier D ....................................................................... 8 Figure S 2: Chormatographic seperation of the cations Na+and Ca2+(a) and the anions Cl-, NO3- and SO42-via ion chromatography. .................................................................................. 11 Figure S 3: Content of (a) Na+, (b) NH4+, (c) Ca2+, (d) Cl-, (e) NO3- and (f) SO42- in selected production batches of AOT of supplier A1, B, C and D displayed as box-plots. .................... 12 Figure S 4: Chromatographic separation of the light-aromatic naphtha solvent in AOT, shown in (a) are the earlier eluting and in (b) the late eluting compounds. ......................................... 17 Figure S 5: Comparison of the chromatographic pattern of the light-aromatic naphtha solvent of selected production batches of AOT of the suppliers A1, C and D. The analysis of the solvent was conducted on GC-MS ........................................................................................... 20 2 List of Tables Table S 1: Retention time and exact masses for compounds in the test sample for checking on mass calibration .......................................................................................................................... 4 Table S 2: Content of diester 1 and monoester 2 and 3 in AOT together with their expanded measurement uncertainty. Analysis of five independently weight samples each batch number averaged. The expended measurement uncertainty is calculated according to GUM encompassing 95% of the distribution of values [1] .................................................................. 6 Table S 3: Content of diester 1, monoester 2 and monoester 3 in sediment given as percentage of the AOT-content in the formulation. The sediment was obtained after centrifugation of the modell agrochemical formulation containing AOT of supplier A1. Each value is the average of five replicates analyses, given together with its interval of confidence of 95%. ................... 9 Table S 4: Content of Na+, Ca2+, Cl-, NO3- and SO42-in selected production batches of AOT of supplier A1, supplier B, supplier C and supplier D. Those ions, which contents were below the LOQ of the used method were indicated with “<LOQ”. .................................................... 12 Table S 5: Compounds in the light-aromatic naphtha solvent in AOT, which were identified via spectra library. Shown are the most likely hits according to retention time and spectrum. 18 3 1. Sample for testing on mass calibration of ToF-MS The retention times and exact masses for the compounds in the test sample for checking on mass calibration of the used ToF-MS are given in Table S 1. Table S 1: Retention time and exact masses for compounds in the test sample for checking on mass calibration Compound Imidacloprid Thiacloprid Tebuconazole (1.Isomer) Triadimenol Tebuconazole (2.Isomer) Distyrylethoxylate-5-EO Distyrylethoxylate-6-EO Distyrylethoxylate-7-EO Distyrylethoxylate-8-EO Distyrylethoxylate-9-EO Distyrylethoxylate-10-EO Distyrylethoxylate-11-EO Distyrylethoxylate-12-EO Distyrylethoxylate-13-EO Distyrylethoxylate-14-EO Distyrylethoxylate-15-EO Distyrylethoxylate-16-EO Distyrylethoxylate-17-EO Distyrylethoxylate-18-EO Distyrylethoxylate-19-EO Distyrylethoxylate-20-EO Distyrylethoxylate-21-EO Distyrylethoxylate-22-EO Distyrylethoxylate-23-EO Distyrylethoxylate-24-EO Distyrylethoxylate-25-EO Distyrylethoxylate-26-EO Distyrylethoxylate-27-EO Distyrylethoxylate-28-EO Distyrylethoxylate-29-EO Distyrylethoxylate-30-EO Nonylphenolethoxylate-5-EO Nonylphenolethoxylate-6-EO Nonylphenolethoxylate-7-EO Nonylphenolethoxylate-8-EO Nonylphenolethoxylate-9-EO Nonylphenolethoxylate-10-EO Nonylphenolethoxylate-11-EO Nonylphenolethoxylate-12-EO Nonylphenolethoxylate-13-EO Nonylphenolethoxylate-14-EO Nonylphenolethoxylate-15-EO tN/min 2.0 2.5 4.3 4.6 4.9 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 6.6 6.3 6.2 6.2 6.2 6.2 6.2 6.2 6.2 6.2 6.2 Exact mass/amu 254.0450 252.0236 307.1451 295.1088 307.1451 522,2981 566,3244 610,3506 654,3768 698,4030 742,4292 786,4554 830,4816 874,5079 918,5341 962,5603 1006,5865 1050,6127 1094,6389 1138,6651 1182,6914 1226,7176 1270,7438 1314,7700 1358,7962 1402,8224 1446,8486 1490,8749 1534,9011 1578,9273 1622,9535 440,3138 484,3400 528,3662 572,3924 616,4186 660,4449 704,4711 748,4973 792,5235 836,5497 880,5759 4 Nonylphenolethoxylate-16-EO Nonylphenolethoxylate-17-EO Nonylphenolethoxylate-18-EO Nonylphenolethoxylate-19-EO Nonylphenolethoxylate-20-EO Nonylphenolethoxylate-21-EO Nonylphenolethoxylate-22-EO Nonylphenolethoxylate-23-EO Nonylphenolethoxylate-24-EO Nonylphenolethoxylate-25-EO Nonylphenolethoxylate-26-EO Nonylphenolethoxylate-27-EO Nonylphenolethoxylate-28-EO Nonylphenolethoxylate-29-EO Nonylphenolethoxylate-30-EO Tristyrylethoxylate-5-EO Tristyrylethoxylate-6-EO Tristyrylethoxylate-7-EO Tristyrylethoxylate-8-EO Tristyrylethoxylate-9-EO Tristyrylethoxylate-10-EO Tristyrylethoxylate-11-EO Tristyrylethoxylate-12-EO Tristyrylethoxylate-13-EO Tristyrylethoxylate-14-EO Tristyrylethoxylate-15-EO Tristyrylethoxylate-16-EO Tristyrylethoxylate-17-EO Tristyrylethoxylate-18-EO Tristyrylethoxylate-19-EO Tristyrylethoxylate-20-EO Tristyrylethoxylate-21-EO Tristyrylethoxylate-22-EO Tristyrylethoxylate-23-EO Tristyrylethoxylate-24-EO Tristyrylethoxylate-25-EO Tristyrylethoxylate-26-EO Tristyrylethoxylate-27-EO Tristyrylethoxylate-28-EO Tristyrylethoxylate-29-EO Tristyrylethoxylate-30-EO 6.2 6.2 6.2 6.2 6.2 6.2 6.2 6.2 6.2 6.2 5.9 5.9 5.9 5.9 5.9 5.9 5.9 5.9 6.5 5.9 5.9 6.0 6.0 6.0 6.0 6.0 6.0 6.0 6.0 6.0 6.0 5.9 5.9 5.9 5.9 5.9 5.8 5.8 5.8 5.8 5.8 924,6022 968,6284 1012,6546 1056,6808 1100,7070 1144,7332 1188,7594 1232,7857 1276,8119 1320,8381 1364,8643 1408,8905 1452,9167 1496,9429 1540,9692 626,3607 670,38695 714,4132 758,4394 802,4656 846,4918 890,5180 934,5442 978,5705 1022,5967 1066,6229 1110,6491 1154,6753 1198,7015 1242,7278 1286,7540 1330,7802 1374,8064 1418,8326 1462,8588 1506,8850 1550,9113 1594,9375 1638,9637 1682,9899 1727,0161 5 2. Content of diester 1, monoester 2 and monoester 3 in different production batches of commercially available AOT of different suppliers In Table S 2 were given the content of diester 1 and the monoesters 2 and 3 in AOT of at least eight production batches each investigated supplier A, B, C and D. The given data for each production batch are average values of five independently weighed repetition analyses after the removal of outliers with a Grubbs outlier test. The displayed data is given together with its interval of confidence of 95%. Table S 2: Content of diester 1 and monoester 2 and 3 in AOT together with their expanded measurement uncertainty. Analysis of five independently weight samples each batch number averaged. The expended measurement uncertainty is encompassing 95% of the distribution of values. Sample [SupplierBatch No.] a-1 a-2 a-3 a-4 a-5 a-6 a-7 a-8 AOT (w/w /%) Monoester 2 (w/w /%) Monoester 3 (w/w /%) 62.9 ± 1.2 58.6 ± 1.2 60.2 ± 0.6 61.3 ± 3.3 62.4 ± 2.1 61.2 ± 0.9 62.6 ± 1.2 62.2 ± 1.1 1.3 ± 0.02 1.5 ± 0.04 1.7 ± 0.02 1.2 ± 0.05 2.0 ± 0.04 1.3 ± 0.01 1.5 ± 0.03 1.3 ± 0.03 0.72 ± 0.02 0.58 ± 0.01 0.93 ± 0.01 0.48 ± 0.02 0.82 ± 0.03 0.72 ± 0.01 0.83 ± 0.01 0.69 ± 0.01 A-1 A-2 A-3 A-4 A-5 64.5 ± 1.0 57.8 ± 1.0 58.0 ± 1.6 56.3 ± 1.0 60.6 ± 0.6 2.8 ± 0.02 2.3 ± 0.05 2.6 ± 0.05 2.4 ± 0.04 2.5 ± 0.08 1.7 ± 0.03 2.1 ± 0.05 2.0 ± 0.04 1.9 ± 0.01 1.8 ± 0.05 B-1 B-2 B-3 B-4 B-5 B-6 B-7 B-8 65.8 ± 0.7 65.0 ± 3.5 65.3 ± 2.1 73.1 ± 1.3 61.3 ± 1.1 62.1 ± 0.7 63.0 ± 1.0 71.3 ± 1.0 0.82 ± 0.01 0.58 ± 0.02 0.80 ± 0.02 1.2 ± 0.03 1.3 ± 0.04 1.0 ± 0.01 0.88 ± 0.01 1.2 ± 0.03 0.15 ± 0.004 0.26 ± 0.01 0.15 ± 0.003 0.36 ± 0.01 0.28 ± 0.02 0.31 ± 0.01 0.21 ± 0.01 0.30 ± 0.01 6 C-1 C-2 C-3 C-4 C-5 C-6 C-7 C-8 61.4 ± 1.0 58.8 ± 0.7 55.7 ± 0.9 62.9 ± 0.6 60.1 ± 0.7 59.0 ± 0.8 57.1 ± 0.9 58.7 ± 0.9 3.2 ± 0.06 2.5 ± 0.06 3.4 ± 0.02 2.5 ± 0.05 3.3 ± 0.05 2.3 ± 0.04 2.4 ± 0.04 2.4 ± 0.03 0.67 ± 0.03 1.0 ± 0.02 1.0 ± 0.02 1.5 ± 0.03 0.73 ± 0.02 0.60 ± 0.01 0.53 ± 0.01 0.54 ± 0.01 D-1 D-2 D-3 D-4 D-5 D-6 D-7 D-8 D-9 D-10 D-11 D-12 D-13 D-14 D-15 D-16 63.9 ± 0.3 61.6 ± 1.1 64.8 ± 1.0 65.1 ± 0.9 64.1 ± 0.7 61.2 ± 1.3 64.6 ± 0.2 64.2 ± 1.0 65.0 ± 1.0 64.4 ± 0.5 65.3 ± 0.7 65.2 ± 0.4 65.2 ± 0.8 60.9 ± 0.7 63.3 ± 0.4 62.5 ± 0.8 3.8 ± 0.09 3.4 ± 0.11 4.1 ± 0.06 4.0 ± 0.09 3.9 ± 0.08 4.1 ± 0.06 3.9 ± 0.05 3.8 ± 0.03 4.0 ± 0.03 3.1 ± 0.08 3.2 ± 0.07 3.0 ± 0.06 2.8 ± 0.09 2.9 ± 0.21 2.9 ± 0.05 3.3 ± 0.05 2.7 ± 0.09 2.4 ± 0.03 2.7 ± 0.08 2.5 ± 0.04 2.3 ± 0.07 2.8 ± 0.04 2.0 ± 0.07 2.3 ± 0.03 2.0 ± 0.03 2.0 ± 0.05 2.2 ± 0.05 2.1 ± 0.04 1.9 ± 0.05 1.8 ± 0.09 2.0 ± 0.04 2.2 ± 0.06 7 3. Sedimentation in trail storage formulation samples The observed sediment in the formulation samples after storage was photographed from above and shown in Figure S 1. Figure S 1: Test on sedimentation after 0.5 a storage at room temperature of a model agrochemical formulation containing AOT of supplier A1, B and D. Increasing amount of visible sediment from supplier A1 to supplier D 4. Centrifugation of a model agrochemical formulation containing AOT of supplier A1 A model agrochemical formulation containing AOT of supplier A1 was centrifuged with a HEREAUS Labofuge 400 with 3000 r/min. The supernatant was removed and the sediment analyzed on diester 1 and monoester 2 and monoester 3. The results of the analyses given as percentage of the AOT-content in the formulation are shown in Table S 3. Each value is the average of five replicate analyses given together with its interval of confidence of 95%. 8 Table S 3: Content of diester 1, monoester 2 and monoester 3 in sediment given as percentage of the AOTcontent in the formulation. The sediment was obtained after centrifugation of the model agrochemical formulation containing AOT of supplier A1. Each value is the average of five replicates analyses, given together with its interval of confidence of 95%. Sediment sample AOT (w/w /%) 236.0 ± 36.2 Monoester 2 (w/w /%) 1.8 ± 0.1 Monoester 3 (w/w /%) 0.9 ± 0.08 5. Results of the analysis of AOT of different production batches for inorganic anions and cations of different suppliers Selected production batches of AOT of supplier A1, B, C and D were investigated on difference in their content of inorganic cations and anions, which are known to influence both ionic and non-ionic surfactants [1;2]. The samples were screened on the content of the cations Li+ Na+, NH4+, K+, Mg2+ and Ca2+, as well as, the anions of Br-, Cl-, F-, NO3-, PO43- and SO42-. Variations in the content of inorganic ions between the suppliers of AOT may explain the differences observed in sedimentation behavior after storage of a model agrochemical formulation containing AOT of either supplier A1, B or D. Analysis was conducted on an ICS 2000 ion chromatography instrument from Dionex. Chromatographic separation of the cations was performed with an IonPa CS12A column (250 x 2.0 mm). For mobile phase methanesulfonic acid (MSA) was taken. The sample was injected with a volume of 5.0 µL and gradient elution was applied for separation of the target analytes. Starting with a concentration of 30 mM MSA and raised to 40 mM in 10 min, lowered to 30 mM MSA in 1.0 min to 30mM MSA by column flushing and equilibration afterwards. Total run time was 15 min with a flow of 0.25 mL/min and a column temperature of 30°C. 9 For chromatographic separation of the anions an IonPac AS11 HC column (250 mm x 2.0 mm) was used. As mobile phase water plus 30mM KOH was taken. The sample was injected with 2.5 µL and the target analytes were eluted isocratically. Total run time was 15 min with a flow of 0.38 mL/min and column temperature of 30 °C. For detection an electrochemical detector connected upstream with a suppressor was used. For analysis of the cations Dionex Six Cation-II Standard was used, containing lithium (c(Li+) = 50 mg/L), sodium (c(Na+) = 201 mg/L), ammonium (c(NH4+) = 251 mg/L), potassium (c(K+) = 501 mg/L), magnesium (c(Mg2+) = 250 mg/L) and calcium (c(Ca2+) = 50 mg/L). This solution had to be further diluted by 1:10 (v/v) diluted to obtain the stock solution for the analysis of cations. For the analysis of the anions a commercially available multi-element ion chromatography anion standard supplied by Fluka was used as standard solution containing, bromide (c(Br-) = 20 mg/L), chloride (c(Cl-) = 10 mg/L), fluoride (c(F-) = 3 mg/L), nitrate (c(NO3-) = 20 mg/L), phosphate (c(PO43-) = 20 mg/L) and sulfate (c(SO42-) = 20 mg/L). For preparation of the standard solutions the both stock solutions were diluted to fit the concentration range 20 mg/L to 1 mg/L. 10 For analysis the light aromatic solvent in AOT was evaporated. An amount of 100 mg of the remainder was diluted with 50 mL of a mixture of 95/5 (v/v) water/methanol. The obtained solution could be directly injected without further dilution accepted for the analysis of Na+, where the sample solution had to be diluted 1:10 (v/v) to be inside the linear range. Of all investigated inorganic ions only the contents of Na+, Ca2+, Cl-, NO3- and SO42- were above the limit of quantification (LOQ) of 1 mg/L of the used analytical method. As this LOQ corresponds to a content of 0.05 % (w/w) in AOT with the given sample preparation, no further attempts were made to detect the other inorganic ions screened for, as their content was considered negligible. In Figure S 2 is shown the chromatographic separation of the target cation (a) and anions (b) for the analysis of the production batch a-1. (a) (b) Figure S 2: Chromatographic separation of the cations Na+ and Ca2+(a) and the anions Cl-, NO3- and SO42via ion chromatography. 11 The obtained results are shown in Table S 4 and are visualized as box-plots in were indicated with “<LOQ”. Sample [Supplier-Batch No.] a-1 a-2 a-3 a-4 a-5 a-6 a-7 a-8 Na+ (w/w /%) 4.7 5.3 5.2 7.5 5.1 3.8 3.7 4.8 Ca2+ (w/w /%) 0.07 Cl(w/w /%) NO3(w/w /%) SO42(w/w /%) <LOQ < LOQ 0.06 0.08 0.05 0.06 < LOQ < LOQ < LOQ < LOQ 0.05 0.09 0.07 0.08 < LOQ < LOQ < LOQ 0.5 0.3 0.6 0.4 0.7 0.4 0.3 0.5 <LOQ 0.1 0.1 0.1 <LOQ 0.08 B-1 B-2 B-3 B-4 B-5 B-6 B-7 B-8 5.0 5.2 4.8 5.4 4.9 5.3 5.2 5.4 <LOQ <LOQ <LOQ <LOQ <LOQ <LOQ <LOQ <LOQ 0.09 0.06 0.06 0.16 0.06 0.14 0.14 0.14 0.1 0.08 0.07 0.2 0.07 0.1 0.1 0.2 0.4 0.3 0.3 0.5 0.5 0.5 0.6 0.5 C-1 C-2 C-3 C-4 C-5 C-6 C-7 C-8 5.7 3.0 4.5 3.5 6.0 4.4 4.9 5.9 <LOQ < LOQ < LOQ < LOQ 0.05 0.06 < LOQ < LOQ < LOQ < LOQ < LOQ 0.05 0.07 0.08 0.05 < LOQ < LOQ 0.3 0.2 0.3 0.3 0.4 0.3 0.4 0.4 D-1 D-2 D-3 D-4 D-5 D-6 D-7 D-8 D-9 D-10 D-11 D-12 D-13 D-14 D-15 D-16 4.2 6.9 5.9 6.8 5.7 5.5 5.8 2.7 3.8 5.3 5.5 4.7 5.7 5.7 5.6 5.3 0.1 0.1 0.07 0.09 0.1 0.06 0.05 0.2 < LOQ 0.05 0.1 0.1 0.07 0.05 0.1 0.08 0.05 0.05 0.05 0.2 0.06 0.06 0.07 0.07 < LOQ < LOQ < LOQ < LOQ < LOQ 0.05 0.05 0.1 0.05 0.05 0.05 0.1 0.08 0.08 0.4 0.4 0.3 0.4 0.3 0.3 0.3 0.3 0.3 0.3 0.4 0.3 0.6 0.4 0.5 0.4 0.2 0.2 0.09 0.1 0.08 0.07 <LOQ <LOQ 0.08 0.05 0.2 0.1 0.1 0.07 0.3 0.2 0.1 12 a) (b) 13 (c) (d) 14 (e) Figure S 3 (a) for Na+, in (b) for Ca2+, in (c) for Cl-, in (d) for NO3- and in (e) for SO42-. Those ions, which contents were below the LOQ of the used method, were indicated with “<LOQ” and were not considered for the box-plot figures. Table S 4: Content of Na+, Ca2+, Cl-, NO3- and SO42-in selected production batches of AOT of supplier A1, supplier B, supplier C and supplier D. Those ions, which contents were below the LOQ of the used method were indicated with “<LOQ”. Sample [Supplier-Batch No.] a-1 a-2 a-3 a-4 a-5 a-6 a-7 a-8 B-1 B-2 B-3 B-4 Na+ (w/w /%) 4.7 5.3 5.2 7.5 5.1 3.8 3.7 4.8 5.0 5.2 4.8 5.4 Ca2+ (w/w /%) 0.07 Cl(w/w /%) NO3(w/w /%) SO42(w/w /%) <LOQ < LOQ 0.06 0.08 0.05 0.06 < LOQ < LOQ < LOQ < LOQ 0.05 0.09 0.07 0.08 < LOQ < LOQ < LOQ 0.5 0.3 0.6 0.4 0.7 0.4 0.3 0.5 <LOQ <LOQ <LOQ <LOQ 0.09 0.06 0.06 0.16 0.1 0.08 0.07 0.2 0.4 0.3 0.3 0.5 <LOQ 0.1 0.1 0.1 <LOQ 0.08 15 B-5 B-6 B-7 B-8 4.9 5.3 5.2 5.4 <LOQ <LOQ <LOQ <LOQ 0.06 0.14 0.14 0.14 0.07 0.1 0.1 0.2 0.5 0.5 0.6 0.5 C-1 C-2 C-3 C-4 C-5 C-6 C-7 C-8 5.7 3.0 4.5 3.5 6.0 4.4 4.9 5.9 <LOQ < LOQ < LOQ < LOQ 0.05 0.06 < LOQ < LOQ < LOQ < LOQ < LOQ 0.05 0.07 0.08 0.05 < LOQ < LOQ 0.3 0.2 0.3 0.3 0.4 0.3 0.4 0.4 D-1 D-2 D-3 D-4 D-5 D-6 D-7 D-8 D-9 D-10 D-11 D-12 D-13 D-14 D-15 D-16 4.2 6.9 5.9 6.8 5.7 5.5 5.8 2.7 3.8 5.3 5.5 4.7 5.7 5.7 5.6 5.3 0.1 0.1 0.07 0.09 0.1 0.06 0.05 0.2 < LOQ 0.05 0.1 0.1 0.07 0.05 0.1 0.08 0.05 0.05 0.05 0.2 0.06 0.06 0.07 0.07 < LOQ < LOQ < LOQ < LOQ < LOQ 0.05 0.05 0.1 0.05 0.05 0.05 0.1 0.08 0.08 0.4 0.4 0.3 0.4 0.3 0.3 0.3 0.3 0.3 0.3 0.4 0.3 0.6 0.4 0.5 0.4 0.2 0.2 0.09 0.1 0.08 0.07 <LOQ <LOQ 0.08 0.05 0.2 0.1 0.1 0.07 0.3 0.2 0.1 16 a) (b) 17 (c) (d) 18 (e) Figure S 3: Content of (a) Na+, (b) NH4+, (c) Ca2+, (d) Cl-, (e) NO3- and (f) SO42- in selected production batches of AOT of supplier A1, B, C and D displayed as box-plots. As shown the content of the investigated inorganic ions, Na+, NH4+, K+, Mg2+, Ca2+, Cl-, NO3and SO42-in AOT was not different between the supplier A1, B, C and D. Therefore the observed differences in the physico-chemical properties of a model agrochemical formulation, containing AOT of either supplier A1, B or D, could not be explained by differences in the content of inorganic ions. 6. Analysis of the composition of the solvent in AOT on differences between the suppliers Selected production batches of supplier A1, C and D were analyzed via GC-MS, to investigate, if there are differences in the composition of the light-aromatic naphtha solvent in which the actual surfactant of AOT, diester 1, is solved in, between the different suppliers. 19 The analysis was performed via gas chromatography coupled to mass spectrometry with electron impact ionization on an Agilent 5973 GC/MS. The sample was injected with 0.2 µL, with a split of 1:60 (GC:waste) on a HP-5 capillary column of Agilent with an inner diameter of 0.18 mm, a length of 20 m and film thickness of 0.18 mm. Separation of the analytes was achieved with a temperature gradient, starting with 60 °C, raising temperature to 200 °C in 28 min. For column cleaning the temperature was then raised to 280 °C in 4 min and held for 3 min at 280 °C. Total run time was 35 min with N2-gas stream set at 150 kPa constant pressure. The Inlet temperature was set at 260 °C, the aux temperature at 280 °C, the temperature in the MS inlet at 250°C and in the MS quadrupole at 150 °C. An amount of 20 mg each AOT sample was solved in 50 mL of a mixture of 1:1 (v/v) ACN/H2O. The obtained solution was then injected into the GC-MS, without further dilution or treatment. The main components of the light-aromatic naphtha solvent was chromatographically separated and identified via a spectra library. The chromatographic separation is shown in Figure S 4 (a) for the early eluting and in Figure S 4 (b) for the late eluting compounds. The most likely hit regarding retention time and spectrum for the main components are displayed in 20 Table S 5. (a) (b) Figure S 4: Chromatographic separation of the light-aromatic naphtha solvent in AOT, shown in (a) are the earlier eluting and in (b) the late eluting compounds. 21 Table S 5: Compounds in the light-aromatic naphtha solvent in AOT, which were identified via spectra library. Shown are the most likely hits according to retention time and spectrum. Retention time [min] 2.48 2.79 3.15 3.30 3.42 3.95 3.99 4.23 4.43 4.66 4.69 4.77 4.83 4.88 4.98 5.18 5.34 5.97 6.06 7.19 8.99 29.24 30.24 Compound 1,3-dimethyl-benzene (1-methylethyl)-benzene Propyl-benzene 1-ethyl-3-methyl-benzene 1-ethyl-2-methly-benzene (2-methylpropyl)-benzene (1-methylpropyl)-benzene 1, 2, 3-trimethylbenzene Indane 1, 3-diethyl-benzene 1-methly-3-propyl-benzene Diethyl-benzene 4-ethyl-1,2-dimethyl-benzene 1, 2-diethyl-benzene 1-methly-4-propyl-benzene 2-ethyl-1, 4-dimethyl-benzene 2-ethyl-1 ,3-dimethyl-benzene 1, 2, 4, 5-teramethly-benzene 1, 2, 3, 4-teramethly-benzene alpha, 4-diemethyl-benzene-methanol 6-methylheptyl ester 2- propionic acid Bis(2-ethylhexyl) maleate 1 ,2-Cyclohexanedione As shown the main compounds identified are benzyl derivates of benzene, which confirms the characterization of the light-aromatic naphtha solvent by its supplier [3;4]. 8 different production batches each supplier A1, C and D were analyzed accordingly, on the composition of their light-aromatic solvent. Exemplary, are given in Figure S 5 the results for one production batch of AOT each supplier, as variations between the analyzed production batches for suppliers were not detected. Shown are separately the range of time 0-10 min in A1-1, C-1 and D-1 and the time range 10-35min in A1-2, C-2 and D-2. 22 (A1-1) (A1-2) (C-1) 23 (C-2) (D-1) (D-2) Figure S 5: Comparison of the chromatographic pattern of the light-aromatic naphtha solvent of selected production batches of AOT of the suppliers A1, C and D. Shown are separately the retention time range 0-10 min (A1-1), C-1 and D-1) and 10-35 min (A1-2, C-2 and D-2). The analysis of the solvent was conducted on GC-MS The compounds listed in 24 Table S 5 were found for all three suppliers. Observed were, however, differences between the investigated suppliers of AOT regarding the abundance of some compounds in the retention time window 2.0-7.0 min. 7. Statistical evaluation of the usefulness of the contents of diester 1 and monoester 2 and 3 for product identification After having analyzed the content of diester 1, monoester 2 and monoester 3 in AOT samples from production batches of different suppliers the question arose if in the future such analytical data could be potentially helpful for identifying the supplier from which an unknown sample originates. The corresponding statistical analysis was performed with R, a language and environment for statistical computing and graphics [5]. Therefore two further documents are provided in the Supporting Information. The first one is the document "evaluation.pdf" which explains the statistical evaluation in detail. The second one is a standalone R script (evaluation.R) which can be immediately executed by the reader. This needs the input files "data_set_1.txt" (samples from batches of various suppliers) and "data_set_2.txt" (trial storage formulation samples) which are also included in the Supporting Information. Reference List 1. Porter MR (1994) Handbook of Surfactants. vol. 2 Chapman & Hall, Glasgow 2. Tadros TF (2008) In: Applied Surfactants, Principles and Applications. Wiley-VCH, Weinheim. 3. Shell Chemicals (Accesed: December 2013) Material Safety data sheat ShellSol A100 http://aglayne.com/wp-content/uploads/2010/10/Shellsol-A-100.pdf. 4. Exxon Mobil Chemical (Accesed: December 2013) Material safety data sheat Solvesso 100 https://www.exxonmobilchemical.com/Chem-English/Files/Resources/aromatic100-product-safety-summary.pdf 5. R Development Core Team (2012) R: A Language and Enviroment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3-90005107-0. 25