Determining the % mass of copper in brass
advertisement

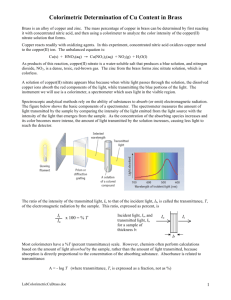
Determining the % mass of copper in brass You can write these directly in your lab notebook as pre-lab calculations. The only other thing you need in your lab notebook would be the purpose. The rest we will work through in class. P.S. The lab manuals arrived at 2:19 in the main office. Lab Homework: 1) Copper metal will be oxidized to copper (II) ions. Nitric acid will be reduced to nitrogen dioxide gas. Write the balanced equation using the half reaction method. 2) Pick a mass of brass you would like to start out with (between 1-2 grams). Calculate the volume (in mL) of 15.8 M nitric acid needed to completely react with your mass of brass (assuming the brass is 100% copper). 3) Pick a wavelength at which you will make a calibration curve that compares various concentrations of Cu(NO3)2 (see #4) with absorbance. You will need to you’re the graphs you received in class. You may also find them on my website with the ch. 4 stuff. Abs = - log10 T (T = % transmittance expressed as a decimal) Abs= 0 means 100% of light reaches the detector. Abs = 1 means 10% of light reaches the detector. Abs = 2 means 1% of light reaches the detector. Abs = 3 means 0.1% of light reaches the detector. You want to pick a wavelength where the absorbance will be greater than zero, but less than 1. 4) You will obtain 10.0 mL of 0.400 M Cu(NO3)2. Determine what volume of is required to make a. 10.00 mL of 0.200 M Cu(NO3)2 b. 10.00 mL of 0.100 M Cu(NO3)2 c. 10.00 mL of 0.0500 M Cu(NO3)2 d. 10.00 mL of 0.0250 M Cu(NO3)2 Note: Read pages A16-A19 to learn more about Spectral Analysis (e.g. Spectrophotometry).