PCR - OIE
advertisement

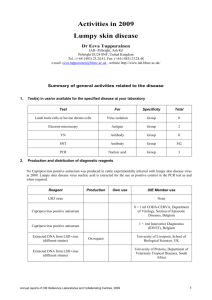
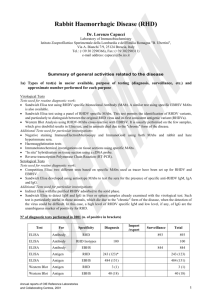
OIE Reference Laboratory Reports Activities in 2011 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Address of laboratory: Central Veterinary Institute of Wageningen UR P.O. Box 65 8200AB Lelystad THE NETHERLANDS Tel.: +31 320 238800 Fax: +31 320 238668 e-mail address: website: Name (including Title and Position) of Head of Laboratory (Responsible Official): Name(including Title and Position) of OIE Reference Expert: Name (including Title and Position) of writer of this report (if different from above): Annual reports of OIE Reference Centres, 2011 Aujeszky’s disease (Pseudorabies) andre.bianchi@wur.nl www.cvi.wur.nl Willie Loeffen, DVM, PhD Project leader CSF, ASF and Aujeszky’s disease Andre Bianchi, PhD Director of CVI Willie Loeffen 1 Aujeszky’s disease Part I: Summary of general activities related to the disease 1. Test(s) in use/or available for the specified disease/topic at your laboratory Test For Specificity Total ELISA Antibody gE 36 ELISA Antibody gE-confirmation (other domains) 48 ELISA Antibody gB 1125 VN Antibody Whole virus 10 SK6 cell culture Virus isolation Whole virus 0 PCR Genome detection gE 42 PCR Genome detection gB 23 Numbers given are for diagnostic tests on samples received from third parties (mainly surveillance and suspicions). Additional tests were carried out on animal experiments, in the framework of ring trials and validation/accreditation purposes of the tests. 2. Production and distribution of diagnostic reagents All batches of commercially available gE and gB-ELISA kits are being checked before they are allowed to be used by the regional labs in the Netherlands (currently 2 other labs) for large scale surveillance of Aujeszky’s disease. The performance of these regional labs is under constant monitoring by providing 2 internal control sera, to be used in every test, and a proficiency test (33 sera) to be carried out twice per year. In 2011 a request for reference materials was received from the Ukrainian reference lab. Virus and sera were sent to the lab. Part II: Activities specifically related to the mandate of OIE Reference Laboratories 3. International harmonisation and standardisation of methods for diagnostic testing or the production and testing of vaccines a) Establishment and maintenance of a network with other OIE Reference Laboratories designated for the same pathogen or disease and organisation of regular inter-laboratory proficiency testing to ensure comparability of results Participation in the first International Workshop for Aujeszky's disease, Ploufragan, France, 23-24 June 2011, organised by the French OIE laboratory for Aujeszky;s disease. b) Organisation of inter-laboratory proficiency testing with laboratories other than OIE Reference Laboratories for the same pathogens and diseases to ensure equivalence of results In 2011 we participated in three ring trials (serology: gE-ELISA, gB-ELISA, VNT) organized by the VLA (UK). No international ringtrials were organised by our lab. 2 Annual reports of OIE Reference Centres, 2011 Aujeszky’s disease 4. Preparation and supply of international reference standards for diagnostic tests or vaccines We hold in stock a set of reference sera for use in the gE and gB ELISA, as well as a batch of standard virus to be used in a VNT. Furthermore, we hold a set of monoclonal antibodies against a range of epitopes of different PRV glycoproteins. 5. Research and development of new procedures for diagnosis and control None. 6. Collection, analysis and dissemination of epizootiological data relevant to international disease control No PR viruses were isolated from the Netherlands. Based on serological surveillance no suspicions were raised in 2011. 7. Maintenance of a system of quality assurance, biosafety and biosecurity relevant to the pathogen and the disease concerned Main diagnostic tests (PCR and ELISA’s) are accredited according to ISO 17025. Other tests (VI, VNT) and laboratory management are accredited according to ISO 9001. Currently all work with Aujeszky’s disease virus takes place within BSL3 facilities. 8. Provision of consultant expertise to OIE or to OIE Member Countries None. 9. Provision of scientific and technical training to personnel from other OIE Member Countries None. 10. Provision of diagnostic testing facilities to other OIE Member Countries We carry out confirmation diagnostics for the Belgian reference laboratory. Samples for confirmation/exclusion of an Aujeszky’s disease outbreak were received from Finland (oropharyngeal swabs and sera). All samples tested negative in the PCR and the ELISA (both gE and gB). 11. Organisation of international scientific meetings on behalf of OIE or other international bodies None. 12. Participation in international scientific collaborative studies None. 13. Publication and dissemination of information relevant to the work of OIE (including list of scientific publications, internet publishing activities, presentations at international conferences) Presentations at international conferences and meetings Loeffen, W. Aujeszky’s Disease - Recognition of regional laboratories. International Workshop for Aujeszky's disease, Ploufragan, France, 23-24 June 2011. _______________ Annual reports of OIE Reference Centres, 2011 3