Surviving Sepsis, 2013, and Update
advertisement

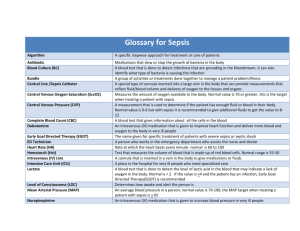
“Surviving Sepsis, 2013, Update” Syllabus Society of Cardiovascular Anesthesiologists Annual Meeting New Orleans, Louisiana March 30, 2014 Gregory E. Kerr MD, MBA, FCCM I. II. III. IV. V. Learning Objectives: At the conclusion of this educational activity, the participants will be able to a. Discuss the new Surviving Sepsis Guidelines b. Describe ongoing controversies of the guidelines No conflict of interest History of the Surviving Sepsis Campaign a. Phase I i. A sepsis definitions conference held December 2001 to determine if new data existed to inform updates to criteria established in 1991. ii. Campaign formed by the SCCM, ESICM, and the ISF and launched at the ESICM Annual Meeting Fall 2002. iii. Survey global public awareness at SCCM Congress 2003 b. Phase II i. 2003 - Reps from 11 societies meet in UK to develop guidelines for management of severe sepsis and septic shock ii. 2004 - Guidelines published in CCM and ICM c. Phase III i. 2003 - Surviving Sepsis Campaign initiated with partnership with IHI. Bundles evolved from collaboration ii. 2004 Importance of American College of Emergency Physicians recognized and they were invited to join campaign iii. 2004 - 2006 - Brochures and manuals disseminated d. Phase IV i. 2013 - ESICM and SCCM announce a reinvigoration of campaign ii. Campaign incorporating new data Definitions a. SIRS b. Sepsis c. Severe Sepsis d. Septic Shock Understanding Recommendations a. GRADE – Grading of Recommendations Assessment, Development and Evaluation b. Quality of evidence from high (A) to very low (D) c. Strength of recommendation from strong (1) to weak or suggestion (2) d. Recommendations divided into three groups VI. i. Those directly targeting sepsis ii. Those directly targeting general care of the critically ill patient and considered high priority in sever sepsis iii. Pediatric considerations Management of Severe Sepsis ( ! – New changes to focus on) a. Initial Resuscitation and Infection Issues i. Initial resuscitation ii. Screening for Sepsis iii. Diagnosis ! 1. Use 1,3 beta-D-glucan assay (grade 2B), mannan and anti-mannan antibody assays (2C), if available and invasive candidiasis is in differential 2. Use low procalcitonin levels or similar biomarkers to in discontinuation of empiric antibiotics in patients who initially appeared septic, but no subsequent evidence of infection (grade 2C) iv. Antimicrobial therapy v. Source control vi. Infection prevention b. Hemodynamic support and adjunctive therapy i. Fluid therapy ! 1. Crystalloids initial fluid of choice (grade 1B) 2. Against hydroxyethyl starches for fluid resuscitation (grade 1B) 3. Albumin for fluid resuscitation when patients need lots of crystalloids (grade 2C) 4. Normalize lactate in patients with elevated lactate levels to target resuscitation (grade 2C) ii. Vaspressor and Inotropic therapy ! 1. Norepinephrine first choice vasopressor (grade 1B) 2. Epinephrine (added to and potentially substituted for norepinephrine) when additional agent needed for blood pressure (grade 2B) 3. Dopamine as alternative vasopressor to norepinephrine only in highly selected patients (grade 2C) 4. Do not use low-dose dopamine for renal protection (grade 1A) 5. Dobutamine infusion be administered or added to vasopressor (if in use) in presence of (a) myocardial dysfunction (b) ongoing signs of hypoperfusion, despite adequate intravascular volume and/or MAP (grade 1C) iii. Corticosteroids! 1. Not using intravenous hydrocortisone to treat adult septic shock patients if adequate fluid resuscitation and vasopressor therapy restore hemodynamic stability VII. VIII. 2. If needed, use iv hydrocortisone alone at dose 200mg/day (grade 2C) c. Supportive therapy i. Blood product administration ii. Immunoglobulins iii. Selenium iv. Mechanical ventilation of sepsis induced ARDS! 1. Use higher PEEP (grade 2C) 2. Use recruitment maneuvers in septic patients with severe refractory hypoxemia (grade 2C) 3. Use prone positioning when PaO2/FiO2 ratio < 100mmHg in facilities with experience (grade 2B) v. Glucose control vi. Renal replacement therapy vii. Stress ulcer prophylaxis viii. Nutrition Additional controversies a. How did Xigris make the initial cut? b. Early goal directed therapy and fluids. Avoiding excess fluids c. Best way to determine optimal hemodyamics Conclusion References: Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management for Severe Sepsis and Septic Shock, Crit Care Med 2013; 41:580–637 http://pulmccm.org/2012/randomized-controlled-trials/xigris-epitaph-prowessshock-results-nejm/ Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/SCCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003 31(4):1250-6 Maitland K, Kiguli S, Opoka RO, et al. Mortality after Fluid Bolus, NEJM 2011; 364(26); 2483-2494 Poole D, Bertolni G, Garrattini S, J Epidemiol Community Health, 2012; 66(7), 571572 Vincent JL, Rhodes A, Perel A, et al. Clinical Review: Update on hemodynamic monitoring – a consensus of 16, Critical Care 2011, 15:229