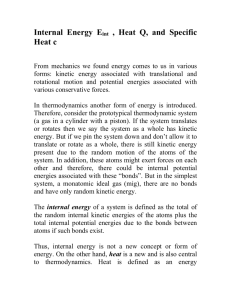
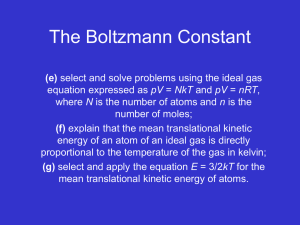
9/16 Introduction to Kinetic Theory and the States of Matter via
advertisement

Kinetic Theory and Phase Changes 9/16 Introduction to Kinetic Theory and the States of Matter via Flipchart Use Kinetic Theory to explain the phase changes in matter via Flipchart 9/17 Plickers Review 9/18 Textbook Reading Guide pages 84-91 Questions 1-6 page 74 Questions 1- 7 page 91 9/21 Textbook Reading Guide pages 75-81 Questions 1-7 page 81 9/22 Reading Quiz (pages 75-91)! Interpreting a Phase Change Graph Phase Change Activity 9/23 Gas Behavior –Temperature, Volume, and Pressure Gas Laws Demonstration/Activity 9/24 Unit Kahoot Review 9/25 Unit Exam Big Ideas: Understand how the energies and motions of atoms and molecules at the microscopic level can be used to understand and predict the macroscopic properties of gases, liquids and solids. Describe how vibrations and waves transport energy. Essential Questions: What are the states of matter? What happens to the atoms of an object when its temperature increases? How can kinetic theory be used to describe what happens to objects when they go through phase changes? Learning Objectives: Students should be able to: Explain how matter changes its state using the kinetic theory Distinguish between exothermic and endothermic processes Standards: ICP.3.1 Describe how we use macroscopic properties of matter to model microscopic processes. ICP. 3.2 Study the characteristics of solids, liquids and gases and their changes of state. Interpret them in terms of a molecular model which describes their energies and motions. ICP. 3.4 Understand how the microscopic kinetic molecular theory explains observations of macroscopic gas behavior in terms of temperature, volume, pressure and the number of particles (using the mole concept). ICP. 4.1 Using conservation of energy, calculate the thermal energy released or absorbed by an object and distinguish between exothermic and endothermic changes. Days 1 and 2: Launch: Students will review the idea that all matter is composed of atoms and that all atoms vibrate and that the faster the atoms vibrate the higher the temperature will be. The teacher will stress that these ideas are the foundation for kinetic theory and that the kinetic theory can be used to explain why matter can change its phase (from gas to liquid to solid). Explore: Students will explore the phase changes of matter (evaporation, freezing, melting, condensation, sublimation, and deposition) via flipchart. Examples using liquid nitrogen and the molecule gloves will be used to illustrate what is happening to the atoms during the phase changes. Summarize: Students will participate in a Plickers review session. This will give the students immediate feedback of their understanding and will serve as a formative assessment. Day 3 Launch: Students will be asked to complete the following activity using the graph shown below. Discussion will follow. Explore: Students will take a closer look at phase changes - observations of water changing from ice to liquid water and finally to water vapor. Class discussion will follow. Summarize: Students will read from the textbook –pages Day 4 Explore: Students will investigate what happens during the gas phase regarding temperature, pressure, and volume. A 2-Liter Pop bottle with a gauge will be used to illustrate how the temperature increases when the pressure increases, but volume stays the same. **Possible Liquid Nitrogen Demo given by the IU Physics Club** Day 5 Summarize: Students will participate in the Unit 9 Jeopardy Review Game Day 6 Summarize: Students will take the Unit 9 Exam