2761-RDWbingo numbers to call and associated questions
advertisement

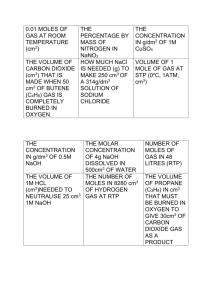
Bingo numbers Bingo questions 240 0.01 MOLES OF GAS AT ROOM TEMPERATURE (cm3) 2 NUMBER OF MOLES OF GAS IN 48 LITRES 24 000 VOLUME OF 1 MOLE OF GAS AT ROOM TEMP (cm3) 22 400 VOLUME OF 1 MOLE OF GAS AT STP (0oC, 1ATM, cm3) 0.08 NUMBER OF MOLES OF O2 IN 200cm3 144 THE VOLUME OF OXYGEN GAS (dm3) NEEDED TO COMPLETELY BURN 180g GLUCOSE (Mr GLUCOSE = 180) 12 THE VOLUME OF GAS IN LITRES PRODUCED WHEN 16g SULFUR COMPLETELY BURNS IN OXYGEN 25 THE VOLUME OF 1M HCL (cm3)NEEDED TO NEUTRALISE 25 cm3 1M NaOH 10 THE VOLUME OF PROPANE (C3H8) IN cm3 THAT MUST BE BURNED IN OXYGEN TO GIVE 30cm3 OF CARBON DIOXIDE GAS AS A PRODUCT 200 159.5 THE VOLUME OF CARBON DIOXIDE (cm3) THAT IS MADE WHEN 50 cm3 OF BUTENE (C4H8) GAS IS COMPLETELY BURNED IN OXYGEN THE CONCENTRATION IN g/dm3 OF 1M CuSO4 20 THE CONCENTRATION IN g/dm3 OF 0.5M NaOH 0.2 THE MOLAR CONCENTRATION OF 4g NaOH DISSOLVED IN 500cm3 OF WATER 78.5 HOW MUCH NaCl IS NEEDED (g) TO MAKE 250 cm3 OF A 314g/dm3 SOLUTION OF SODIUM CHLORIDE 22.33 THE MEAN OF FOUR TITRATIONS: 22.3cm3 30.0cm3 22.4cm3 22.3cm3 3.6 THE MASS OF SODIUM CHLORIDE (g) IN 300 cm3 OF SOLUTION WITH A CONCENTRATION OF 12g/dm3 50 THE CONCENTRATION IN g/dm3 OF 25g COPPER SULFATE DISSOLVED IN 500cm3 WATER 0.345 THE NUMBER OF MOLES IN 8280 cm3 OF HYDROGEN GAS AT RTP 1 THE MOLE CONCENRATION OF 98g/dm3 OF H2SO4 (mol/dm3) 91.25 THE MASS CONCENTRATION OF A 2.5 mol/dm3 SOLUTION OF HCl (g/dm3) 3 THE MOLE CONCENRATION OF 120g/dm3 OF NaOH (mol/dm3) 80 THE MASS CONCENTRATION OF A 2 mol/dm3 SOLUTION OF NaOH (g/dm3) 22.4 22.3 22.4 THE CORRECT BURETTE READING 12 000 THE VOLUME OF CO2 GAS PRODUCED WHEN 50g CaCO3 IS BROKEN DOWN BY HEATING (cm3) RTP 16.5 THE PERCENTAGE BY MASS OF NITROGEN IN NaNO3 0.1 THE CONCENTRATION OF HCl IF 49.8cm3 OF HCl NEUTRALISES 25cm3 OF 0.2 mol/dm3 KOH. 3000 THE VOLUME OF H2 (IN cm3) FORMED WHEN 5g OF CALCIUM REACTS WITH HYDROCHLORIC ACID AT RTP. 168 THE VOLUME (IN LITRES) OF 7 MOLES OF METHANE GAS AT RTP 60 THE VOLUME OF CARBON DIOXIDE (IN dm3)FORMED WHEN 30 dm3 OF OXYGEN REACTS WITH CARON MONOXIDE 0.0125 THE NUMBER OF MOLES IN 25cm3 OF 0.5mol/dm3 CALCIUM HYDROXIDE SOLUTION 5 THE NUMBER OF MOLES OF H2SO4 NEEDED TO NEUTRALISE 10 MOLES OF NaOH 0.025 THE NUMBER OF MOLES OF HYDROCHLORIC ACID IN 50cm3 OF 5M HCl