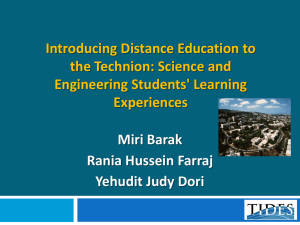
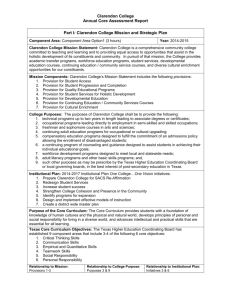
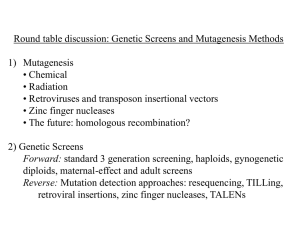
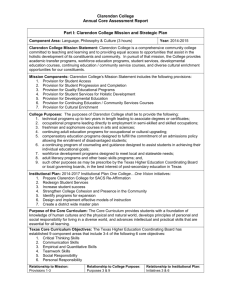
tpj13065-sup-0023
advertisement

Legends for Supporting Information Figure S1. Amino acid sequences of the bHLH region and phylogenetic analysis of DPF and DPF-like proteins. (a) Amino acid sequences of the bHLH region of the DPF protein and rice homologous proteins. Black and gray backgrounds indicate identical and similar amino acids, respectively. Basic HLH domains are indicated below the sequences. Arrows in the basic region indicate the three residues, His (H), Glu (E) and Arg (R), comprising the classic G-box binding region (Toledo-Ortiz et al., 2003). (b) Phylogenetic relationships among the DPF and DPF-like proteins in plants. The corresponding sequence alignment is shown in Figure S1c. Bootstrap values from 1,000 replications are indicated at each node. Hv, Hordeum vulgare; Os, Oryza sativa; Sb, Sorghum bicolor; Ta, Triticum aestivum; Zm, Zea mays. The bar corresponds to 0.1 amino acid substitutions per site. (c) Sequence alignment of DPF (Os01g0196300), DPF-like proteins, and related proteins from various plants. Black and gray backgrounds indicate identical and similar amino acids, respectively. Figure S2. Typical gross morphology and leaf phenotype of DPF overexpressing plants in the vegetative phase. Gross morphology (a) and phenotype of the 3rd youngest leaf blade (b) of the descendants of DPF-OX-1 and -15 lines, and wild-type (WT) eight weeks after sowing. Lesion mimics appeared in the DPF-OX-1 leaves. DPF-OX, DPF overexpressor; WT, wild-type. Figure S3. Lesion mimic phenotype of leaves overexpressing DPF in the ripening phase. Flag leaves of the wild-type (WT), and descendants of DPF-OX-1, -15 and 16 lines. The 1/5 image enclosed by a blue line is a close-up. The lesion mimic phenotype of flag leaves is more severe than that of adult leaf blades in the vegetative phase (Figure S2). Figure S4. Measurement of transcript levels of DPF and the accumulation of diterpenoid phytoalexins in DPF-OX leaves. (a) Relative expression levels of DPF transcripts normalized by RUBQ1. (b) Accumulation levels of MLs-A and B. (c) Accumulation levels of PCs-A to E. The 3rd youngest leaf blades at the heading stage of each T1 plant were sampled and used for the measurement. Three leaf discs were used for the measurement of phytoalexins and the average is shown on the bar. Asterisks indicate that the lesion mimic appeared in the sampled leaf. FW, fresh weight; n.d., not detected; WT, wild-type. Figure S5. Accumulated levels of diterpenoid phytoalexins in DPF-OX husks. (a) Accumulation levels of MLs-A and B. (b) Accumulation levels of PCs-A to E. Husks were sampled from seeds of each harvested T1 plant and used for the measurement. Values are means ± SD of four biological replicates. Asterisks show values that differ significantly from the WT. *P<0.05; **P<0.01 (Student’s t-test). n.d., not detected; WT, wild-type. Figure S6. Expression patterns of DPF-like1 & 2 in wild-type rice. (a) Expression patterns in the leaf blade (LB) and roots in 10-day-old shoots. For LB, RNA was purified from the youngest leaves. Values are means ± SD of three biological replicates. (b) The CuCl2-inducible expression pattern after 24 h treatment. 2/5 Figure S7. Expression patterns of DPF and several genes in MeJA or solvent (mock) treated wild-type rice. (a) Expression pattern of DPF after 12 and 24 h treatments. (b) Expression pattern after 24 h treatment. Values of the expression level of DPF are the same as shown in (a). Values are means ± SD of three biological replicates. Asterisks show values that differ significantly from the mock. *P<0.05; **P<0.01 (Student’s t-test). Figure S8. Expression analyses of representative MEP pathway genes (DXS3, DXR and HDR) and GGPS in the wild-type and two DPF-KD lines. RNA purified from 10-d-old roots was used. Values are means ± SD of three biological replicates. Asterisks show values that differ significantly from the WT. **P<0.01; ***P<0.001 (Student’s t-test). Figure S9. Transient transactivation of promoters of DP biosynthetic genes by DPF. The 2-kb upstream regions of CPS2, KSL4 and CYP99A2 were used as the reporter plasmids. pUCAP was used as a control plasmid. P35S, cauliflower mosaic virus 35S promoter; Tnos, nopaline synthase terminator; hRluc, engineered Renilla luciferase gene (Promega).Values are means ± SD of three biological replicates. Asterisks indicate that the activities with DPF plasmid were significantly higher than that with the control plasmid (*P<0.05, Student’s t-test). Figure S10. Transient transactivation of the CPS2 promoter by DPF. (a) 400-bp upstream region, 400-bp upstream region with mutated G-box element, 400-bp upstream region with mutated G-box-like element, 400-bp upstream region with both elements mutated and the 3/5 259-bp upstream region were used. G-box, 5-CACGTG-3; G-box like, 5-CACGTA-3; N-box, 5-CACGAG-3; M, mutation; hRluc, engineered Renilla luciferase gene (Promega). (b) 259-bp, 205-bp, 150-bp and 95-bp upstream regions were used. Figure S11. Expression analyses of WRKY45 and DPF in wild-type and WRKY45-KD lines. RNA was purified from 10-d-old roots. WT, wild-type; W45-KD, WRKY45-KD. Values are means ± SD of four biological replicates. Asterisks show values that differ significantly from the WT. **P<0.01 (Student’s t-test). Figure S12. Distribution of N-boxes and G-boxes in the 2-kb upstream regions of DP biosynthetic genes. The 5-untranslated region has not been reported; therefore, the 2-kb upstream region from the translational start site is shown in KSL8. Black and white triangles indicate the G-box and N-box, respectively. The asterisk indicates that because an approximately 200-bp unreadable region exists around -700, there may be N-boxes in that region. Table S1. List of genes coexpressed with Os01g0196300 (DPF) by RiceFREND, a rice gene coexpression database Mutual rank is the index for coexpression. Table S2. Primers used in this study 4/5 Method S1. Plant materials Method S2. Phylogenetic analysis Method S3. Isolation and analysis of nucleic acids Method S4. Plasmid construction and rice transformation Method S5. CuCl2 , UV and methyl jasmonate treatments Method S6. Transient expression assay 5/5