4.1 Notes
advertisement

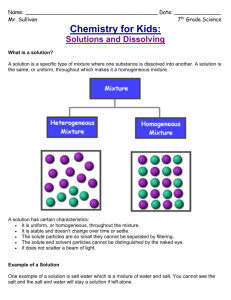
Name: __________________________ Pd. _________ Eff. ______/5 Ch. 4 Sec. 1 Notes A Solution is a Type of Mixture -A __________________ is a combination of substances. -An example of a mixture: _____________________ The parts of a solution are mixed ____________________. _____________________________________________ -The ingredients of any mixture can be separated from each other because they are not ________________________ changed. -Solution: ____________________________________ _____________________________________________ -A solution can be physically separated, but all portions of a solution have the same _____________________. -Examples of solutions: ________________________ _____________________________________________ -Solute: ______________________________________ Solutes and __________________ _____________________________________________ -When a solute dissolves, it separates into ________________ particles. -Solvent: _____________________________________ _____________________________________________ -B/c a solute dissolved into individual particles in a solvent, it is not possible to identify the solute and solvent as different substances when they form a _______________________. -In the solution of table salt and water, which is the solute and which it the solvent? ________________ ‘ _____________________________________________ -Many solutions are made of _________________ dissolving in _________________. -Solvents, solutes, and solutions can be _________, liquids, or solids. -In some solutions, both the solute and solvent are Types of Solutions in the same _________________ state. -Example: ___________________________________ -Example of a solid solution: ___________________ -The air you breathe is a solution! Nitrogen is considered the __________________ whereas; Oxygen and Carbon Dioxide are solutes. -Suspension: _________________________________ Suspension _____________________________________________ -Sometimes you can separate the components of a suspension by ___________________ the mixture. -When a solid dissolves in a liquid, the particles of the solute are surrounded by particles in the ___________________. The solute particles have become evenly _____________________ throughout the solvent. -The way in which a solid compound dissolves in a liquid depends on the type of ___________ in the compound. -In every solution—solid, liquid, and gas—solutes change the physical properties of a ______________ -A solution’s physical properties differ from the physical properties of the ___________ ______________. -freezing point: _______________________________ Solvent and solute particles ______________________. _________________ of solvents change in solutions. Lowering the _________________ point. _____________________________________________ -The freezing point of a liquid solvent ________________--becomes lower—when a solute is __________________ in it. -How can lowering the freezing point in water be useful in the winter? __________________________ _____________________________________________ -Boiling point: ________________________________ Raising the ______________ point. _____________________________________________ -A solution can remain a liquid at a higher temperature than its _____________ solvent. -The more solute that is added, the greater the ________________ in the boiling point. -A solute lowers the ________________ point and raises the boiling point of the solvent in the solution.