Atomic Theory and the Periodic Table
advertisement

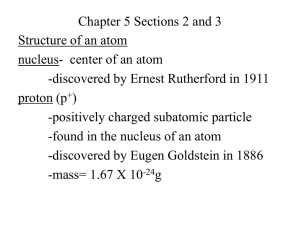
A v. October 09 Atomic Theory and Periodic Table Unit Test DIRECTIONS: TAKE YOUR TIME AND READ THE QUESTIONS SLOWELY. If you have a question, don’t hesitate to raise your hand and ask for help. I might not be able to give you the answer your looking for but it never hurts to ask. MAKE SURE THAT YOU RECORD THE VERSION NUMBER IN THE UPPER LEFT HAND CORNER OF THE QUIZ ON YOUR SCANTRON IN THE BOX THAT SAYS “FORM”. DO NO WRITE ON THIS QUIZ. MARK A FOR TRUE AND B FOR FALSE. 1. In what direction does atomic number increase on the periodic table? a. b. c. d. e. B & C are both true f. B & A are both true 2. How many protons does Potassium have in its nucleus? a. 39 b. 39.1 7. What is the atomic number of Phosphorus? a. 14 b. 31 c. 30.973 d. 5 e. None of the above 8. In what direction on the periodic table does atomic mass increase most of the time? a. b. c. d. c. 19 e. B & C are both true d. 1 f. B & A are both true 3. T or F Iodide has a higher atomic number then Tellurium 4. How many protons does Phosphorus have? a. 30.97 b. 31.0 c. 31 d. 15 5. What is the atomic mass of Iron? a. 26 b. 54 c. 55.8 d. 60 e. None of the above 6. T or F Moving from left to right across the periodic table atomic number always increases. 9. Which of the following elements has an atomic mass larger then Tellurium? a. Indium b. Carbon c. Iodine d. Xenon 10. What element has 17 protons in its nucleus? a. Fluorine b. Oxygen c. Neon d. Chlorine 11. Which of the following elements is NOT a metal? a. Aluminum b. Barium c. Sodium d. Potassium e. Krypton 12. Which one of the following elements is a semi-metal? a. Sodium b. Carbon c. Copper d. Gold 19. Select the element that is not a semi-metal. a. Boron b. Germanium c. Phosphorus d. Tellurium e. Arsenic e. Silicon 13. Is Sulfur a metal, non-metal or semi-metal? a. Metal b. Semi-metal c. Non-metal 14. Which one of the following elements is a metal? a. Oxygen b. Phosphorus c. Chlorine d. Fluorine e. Magnesium 15. What group of elements does Chlorine belong too? a. Noble gases b. Alkaline earth metals c. Halogens d. Semi-metals 16. What group of elements does Helium belong too? a. Non-metals b. c. d. e. f. Metals Semi-metals Halogens Alkali Earth metals None of the above 17. Hydrogen is a… a. Metals b. Semi-metal c. Halogen d. Non-metal e. None of the above. 18. Which of the following elements all belong in the Halogen group? a. Nitrogen, Bromine, Fluorine, & Iodine b. Iodine, Bromine, Chlorine, & Potassium c. Fluorine, Neon, Chlorine, & Sulfur d. Fluorine, Iodine, Bromine, & Chlorine. 20. Which one of the following elements is a metal? a. Chlorine b. Phosphorus c. Silicon d. Iodine e. Potassium 21. Most of the mass in an atom is located where? a. The mass of an atom is spread evenly throughout b. Electron cloud c. Nucleus d. None of the above 22. How much of an atom is empty space? a. 90% b. 10% c. 5% d. 99.999% e. None of the above 23. What is inside of the nucleus? a. Positrons b. Photons c. Electrons d. None of the above 24. Which of the following statements most accurately describes an atom? a. An atom is like plum pudding, all of the mass and charge are spread evenly throughout. b. Most of the space within an atom is located in the nucleus. c. Most of the mass in atom is located in the nucleus, but the nucleus is much smaller then the actual atom itself. d. None of these statements accurately describe an atom. 25. Which of the following sub atomic particles has a mass of 1 amu? a. Protons b. Positrons c. Electrons d. Neutrons e. A & D are both true 26. The ________________ of the atom occupies almost no volume but contains almost all the mass a. Electron cloud b. Valence electron shell c. Core electrons d. nucleus 27. T or F Electrons have a positive charge. 28. Which of the following is NOT a subatomic particle a. Electron b. photon c. Neutron d. Proton 29. T or F Electrons have almost no mass 30. If the atom were a football stadium the nucleus would be… a. A truck inside the stadium b. The goal post inside the stadium c. Ten people inside the stadium d. A marble inside the stadium 31. What is the name of the process that is going on inside of stars? a. Fusion b. Fission c. Combustion d. Burning 32. Where are most of the elements on the periodic table created? a. In laboratories b. They were created immediately after the big bang c. Inside of stars d. In processes occurring on earth 33. ____________ is when one nucleus combines with another nucleus to form a bigger element a. Fission b. Combustion c. Dehydration d. Fusion e. None of the above 34. _____________ is when a very heavy element splits into two smaller elements a. Fission b. Combustion c. Dehydration d. Fusion e. None of the above 35. Which of the following elements existed before the first stars were formed? a. Carbon b. Oxygen c. Sulfur d. Neon e. Hydrogen 36. What process gives us all the energy we have on earth? a. Fission b. Combustion c. Fusion d. Radioactive decay 37. What is the name of the process that is going on inside of nuclear power plants like the one homer simpson works at? a. Fusion b. Fossil Fuel combustion c. Fission d. Nucleosynthesis 38. T or F My body is made of elements that were at one point created inside of a star. 39. Four hydrogen atoms can combine to form a helium atom; this is an example of what? a. Fusion b. Fission c. Endothermic chemical reaction d. None of the above 40. T or F Fusion gives us the energy we have on earth and the higher elements that make up our bodies.