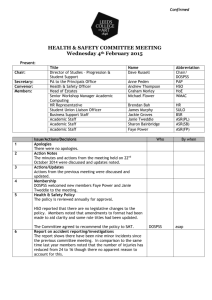
C. elegans exam 1. The mutation lin
advertisement

C. elegans exam
1. The mutation lin-33(j71) causes a multivulva phenotype. The phenotype is due to
the fact Pn.p cells that would normally undergo a 3° fate lineage instead undergo a
2° lineage. These extra 2° cells give rise to the multiple vulva phenotype.
You cross homozygous lin-33(j71) hermaphrodites to wildtype males, single the
mated hermaphrodites and obtain the following progeny: 50 phenotypically normal
males, 45 hermaphrodites. All of these hermaphrodites have multivulvae
phenotype.
a. Is lin-33(j71) recessive or dominant?
Dominant
You then note that j71/+ animals are multivulva but look closer to wild type than j71
homozygotes. Df5 is a deficiency that includes the lin-33 locus. j71/Df5 animals have
a very weak multivulva phenotype and +/Df5 animals are not multivulva.
b. Give a brief explanation.
The j71 allele is a gain-of-function and gene dosage matters. (people may also say
that it is not haploinsufficient).
2.
A common feature of the nervous systems in animals is bilateral symmetry
(e.g. left/right hemispheres of the brain). However, while many structures may be
symmetrical morphologically, they may be asymmetric in terms of their functions.
One such example is provided by left/right asymmetrical gene expression in a pair
of symmetric chemosensory neurons of the worm named the S.E.A. neurons. In the
adult worms, one of the two symmetric neurons lies on the left side of the worm’s
brain SEA/left (SEAL) while the other is on the right SEA/right (SEAR). SEAL and
SEAR can sense different chemical stimuli since they express different
chemoreceptors. For example the chemoreceptor gene gcc7 is only expressed in
SEAL while gcc5 is only expressed in SEAR. These asymmetric expression patterns
can be visualized by generating transgenic animals that express, for instance, GFP
reporters driven by the gcc7 promoter, hence showing up in SEAL (Figure 1).
To elucidate how asymmetric expression of chemoreceptors is established, you
conduct a genetic screen for mutants in which the normally SEAL-specific
expression of gcc7promoter::gfp is disrupted. One mutant derived from this screen,
sym-1(ot1), not only loses gcc7expression but also expresses the normally SEAR
restricted gcc-5 in both SEAR and SEAL (Figure 1).
SEAL
Figure 1
SEAR
gcc-7 promoter:gfp
gcc-5 promoter:gfp
Wild type
sym-1
Animals expressing gcc-7::gfp (%)
Wild type
sym-1(ot1)
100
0
0
100
n=70
n=62
100
0
0
100
n=62
n=53
Animals expressing gcc-7::gfp (%)
Wild type
sym-1(ot1)
You map and clone SYM-1. You then determine that in wild type animals, sym-1 is
normally expressed in SEAL (but not SEAR neurons). You then use a promoter
Ex[ceh-1p], which you already know is expressed in both SEAR and SEAL, to express
wild type sym-1 and obtain the following results:
Animals expressing gcc-5p:gpf (%)
Wild type
sym-1(ot1)
Ex[ceh-1p::sym-1]; sym-1(ot1) (line 1)
Ex[ceh-1p::sym-1]; sym-1(ot1) (line 2)
0
0
92
30
0
0
0
23
100
0
4
19
0
100
4
28
n=62
n=53
n=24
n=43
{rows 3-4 above are two transgenic lines in which wild type sym-1is expressed in sym-1 mutants.
The circles show pattern gcc5p::gfp expression in SEAL and SEAR neurons, white circles: no
expression, green circles: expression; the number below circles indicate how often (%) such patterns
were seen in the total number (n) in which they were counted}
a) Based on the expression data presented so far, what do you hypothesize about
the regulatory relationships between sym-1 and expressions of gcc-5, and gcc-7?
This is what we know: in wild type animals, sym-1 is normally expressed in SEAL
only, where gcc-7 but not gcc-5 is expressed. Loss of sym-1 abrogates expression of
gcc-7 and allows for gcc-5 expression in both neurons. Forced expression of sym-1 in
both neurons dampens the normal expression of gcc-5 in SEAR. One model
consistent with this data is that sym-1 function in SEAL promotes expression of gcc-7
(is required for it) while repressing expression of gcc-5.
b) Instead of examining gcc5p:;gfp, you now examine gcc7p::gfp and find the
following results:
Animals expressing gcc-7p:gpf (%)
Wild type
sym-1(ot1)
Ex[ceh-1p::sym-1]; sym-1(ot1) (line 1)
0
10 0
0
100
0
8
0
0
0
0
0
92
n=52
n=59
n=44
Do these results corroborate or refute your model proposed above?
This corroborates it in that they are consistent with a role of sym-1 in promoting
gcc-7 expression and gcc-5 repression.
c) From your screen, you also find the following sym-2 and sym-3. Each encode for a
distinct gene. Each of the mutants that you have is recessive and represents a null
allele of the gene.
Animals expressing gcc-5p:gpf (%)
Wild type
sym-1(ot1)
sym-2(ot5)
sym-3(ot9)
0
0
100
0
0
0
0
0
100
0
0
0
0
100
0
100
n=54
n=57
n=60
n=60
Animals expressing gcc-7p:gpf (%)
Wild type
sym-1(ot1)
sym-2(ot5)
sym-3(ot9)
0
100
0
0
100
0
3
100
0
0
0
0
0
0
97
0
n=52
n=59
n=60
n=60
You also conduct the following epistasis analyses
Animals expressing gcc-5p:gpf (%)
Wild type
sym-1; sym-2
sym-2; sym-3
0
100
0
0
0
0
100
0
0
0
0
100
n=44
n=67
n=55
Animals expressing gcc-7p:gpf (%)
Wild type
sym-1; sym-2
sym-2; sym-3
0
0
0
100
0
0
0
0
0
0
100
100
n=54
n=47
n=65
sym-1 encodes for a microRNA, sym-2 and sym-3 each encode for a different
transcription factor. Also, in a wild type animal, you normally see sym-2 expression
in SEAR but not SEAL and sym-3 expression in SEAL and not SEAR. Based on these
bits of information, how do you think that the pathway is organized relative to
control of asymmetric expression of the chemoreceptors gcc-5 and gcc-7?
Here is one model that fits all of the data (they may be other models too but they also
need to fit ALL of the data, including the expressions patterns, epistasis, etc.
SEAL
SEAR
sym-1
sym-1 (on)
sym-2 (on)
sym-2
gcc-7 (on)
sym-3 (on)
gcc-5
gcc-7
sym-3
gcc-5 (on)