Environmental Isotope Mini-Poster Extra Credit Resources
advertisement

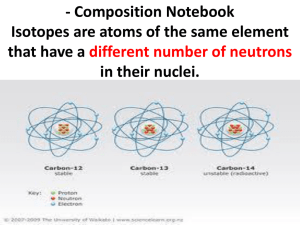
GO HERE for info about specific isotopes, their abundances, and uses of unstable (radioactive) isotopes: http://ie.lbl.gov/education/isotopes.htm A few suggested isotopes: 210Pb (lead-coral growth), 13C (Seuss effect, diets, plant communities, food chains), 14C (dating, Seuss effect), 2H (latitudes, tracers), 18O (glacial periods, temperature, latitude), 13C2 18 H- O “signatures” (geographic locations, migration), 224 Ra (radium, used to detect water input from land ice into Antarctic seawater-email me for link), 15N (studying nitrogen fixation by different organisms, nutritional state of rhinos and moose, food chains) , 33S and 34S versus 32S (sulfur isotopes detecting ancient life in 3 billion year old rocks). Email me if you have questions. Use your own words in your paragraphs. Do NOT duplicate work please. Some Links: CSI Hair and H isotopes: http://www.time.com/time/health/article/0,8599,1720520,00.html 18Oxygen and groundwater: https://gsa.confex.com/gsa/2012AM/webprogrampreliminary/Paper211217.html Tracing lead pollution http://www.sciencedirect.com/science/article/pii/S0160412007001985 Monarch migration: Better migration through chemistry…18O/16O again http://whyfiles.org/083isotope/index.html Deuterium and latitude: http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/isotopes_deuterium.asp Another Hydrogen example http://www.fort.usgs.gov/resources/research_briefs/bat_isotopes.asp see with this http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/isotopes_deuterium.asp Isotope signatures: http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/isotopes_howtheywork.asp http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/ Migratory birds: http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/isotopes_prelimdata.asp Dolphin populations: http://www.fort.usgs.gov/Products/Publications/22917/22917.pdf List of isotope students on salmon, bear, dolphins, etc. http://www.fort.usgs.gov/Research/research_tasks.asp?TaskID=2402 Lead in corals; http://oceanexplorer.noaa.gov/edu/learning/player/lesson15/l15_la1.html Pb-210 Coral growth rate half-life 22 years C-13 and C-14 depletion as indication of fossil fuel contrib. to atmospheric C)2 http://environment.newscientist.com/channel/earth/climate-change/dn11638 Tracking Monarchs -H-2 Deuterium ratios used to trace monarch origins. Hotter less D2O/H2O Isotopes .. better migration through chemistry http://whyfiles.org/083isotope/2.html http://monarchwatch.org/class/studproj/hiso.htm O-18 near equator, O-16 lighter carried to poles. Used to determine temperature moisture during human evolution. Different ratio in transpired and surface water http://earthobservatory.nasa.gov/Features/Paleoclimatology_OxygenBalance/oxygen_balance.php http://climate.nasa.gov/ C-4 and C-3 plants have different C isotope signatures Used to determine diet in early humans and other fossils Nitrogen isotopes – used to detect starvation in rhinos, Isle Royal moose http://ie.lbl.gov/education/isotopes.htm CSEE Interactive Page – must include Isotopes Project Home Page http://ie.lbl.gov/ip.html Excess Ra used to detect terriginous input to ocean water around icebergs Science 317, 478 (2007); Kenneth L. Smith, Jr., et al. Biological Enrichment in the Weddell Sea Free-Drifting Icebergs: Hot Spots of Chemical and Biological Enrichment in the Weddell Sea Excess 224Ra (that fraction not supported by ambient parent isotopes) was chosen as an unambiguous terrigenous input. Because the only viable source of excess 224Ra is the decay of 228Th associated with terrigenous particles, the enrichment must indicate local input of ice-rafted detritus into the surrounding surface waters (typically <10 m depth) from meltwater. The seasurface layer in areas proximal to both icebergs was enriched in excess 224Ra when compared with deeper mixed-layer samples (30 to 80 m depth) that were at or below the detection limit (Fig. 2, E and F), precluding a deep upwelled source of enrichment. Surface enrichments decreased with increasing distance from the icebergs. The highest excess 224Ra enrichment [5.0 disintegrations per minute (dpm) m–3] was observed in water samples collected in brash ice broken off and immediately adjacent to iceberg W-86 (Fig. 2E). STABLE ISOTOPES FOR DUMMIES-good stuff! http://archaeology.about.com/b/2006/10/24/stable-isotopes-for-dummies.htm http://archaeology.about.com/od/stableisotopes/a/si_intro.htm New Applications of Stable Isotope Research Since 1977, applications of stable isotope analysis have exploded in number and breadth, using the stable isotopes ratios of the light elements hydrogen, carbon, nitrogen, oxygen and sulfur in human and animal bone (collagen and apatite), tooth enamel and hair, as well as in pottery residues (baked onto the surface or absorbed into the ceramic wall) to determine diets and water sources. Light stable isotope ratios (usually of carbon and nitrogen) have been used to investigate such dietary components as marine creatures (e.g. seals, fish and shellfish), various domesticated plants such as maize and millet; cattle dairying (milk residues in pottery), and mother’s milk (age of weaning, detected in the tooth row). Dietary studies have been done on hominins from the present day to our ancient ancestors Homo habilis and the Australopithecines. Other isotopic research has focused on the geographic origins of things. Various stable isotope ratios in combination, sometimes including the isotopes of heavy elements like strontium and lead, have been used to determine whether the residents of ancient cities were immigrants or were born locally; to trace the origins of poached ivory and rhino horn to break up smuggling rings; and to determine the agricultural origins of cocaine, heroin, and the cotton fiber used to make fake $100 bills. The latest wrinkle involves the potential use of hydrogen and oxygen isotopes in hair to determine where a person has been lately, by comparing the measurements with an international database for rainfall and local water supplies. Another example of isotopic fractionation that has a useful application involves rain, which contains the stable hydrogen isotopes 1H and 2H (deuterium) and the oxygen isotopes 16O and 18 O. Water evaporates in large quantities at the equator and the water vapor disperses to the north and south. As the H2O falls back to earth, the heavy isotopes rain out first. By the time it falls as snow at the poles, the moisture is severely depleted in the heavy isotopes of hydrogen and oxygen. The global distribution of these isotopes in rain (and tap water) can be mapped and the origins of the consumers can be determined by isotopic analysis of hair. (Watch our for skinheads arriving from the Axis of Evil!) Pass the Sulfur, Please Carbon and sulfur isotopic signatures provide the main evidence for the identification of the earliest life on Earth. Details in the signatures of the several sulfur isotopes can now be used to track metabolism. It was previously suggested that a large fractionation in the 34S versus 32S isotopes implies that sulfate-reducing bacteria were present in rocks dated to about 3.5 billion years ago. Philippot et al. (p. 1534; see the Perspective by Thamdrup), making use of 33S data, show these rocks record the presence of organisms that metabolized and disproportionated elemental sulfur. Several such organisms are present near the base of the phylogenetic tree. This Week in SCIENCE September 14 2007, 317 (5844) http://www.sciencemag.org/content/vol317/issue5844/twis.dtl http://whyfiles.org/083isotope/3.html An Airy Meal The fixation of atmospheric nitrogen into ammonia that is essential to human nutrition and global ecosystems is performed by free-living bacteria and by symbionts in plant root nodules. Lechene et al. (p. 1563; see the Perspective by Kuypers), using multi-isotope imaging mass spectrometry with 15 the stable isotope of N, measured nitrogen fixation by symbiotic bacteria. They traced the utilization of fixed nitrogen, in this case by animal rather than by plant host cells. CREDIT: LECHENE ET AL. How Stable Isotopes Work: Creating a “Signature” http://www.fort.usgs.gov/resources/spotlight/shorebird_isotopes/isotopes_howtheywork. asp Isotopes are forms of a chemical element that have different numbers of neutrons and therefore a different atomic mass. Stable isotopes are those that do not decay with time, and include isotopes of hydrogen, oxygen, nitrogen, carbon, and sulfur. The power of stable isotopes for the study of migratory birds hinges on two important facts. First, the quantity of found in natural according to wellRatios of deuterium, proportion to the annual precipitation, variations across the globe. isotopes, or isotopic ratios, environments varies spatially defined natural processes. for example, vary in temperature and quantity of resulting in well-mapped corresponding to latitude Second, the isotopic ratios in a geographic area are reflected in the local food chains. Hence, an animal’s tissues reflect the isotopic ratios of its foods (such as plant material or insects), which in turn reflect isotopic ratios in the local water and soils. A bird that migrates across geologic areas with large isotopic gradients, such as South America, will exhibit different isotopic concentrations in its various body tissues. The turnover rate of the isotopes differs among tissues; e.g., carbon isotopes have a turnover rate of a few days in liver tissue, a few weeks in muscle and blood, and up to six months in bone collagen. Feathers are unique, however, in that they grow in a very short time and then become metabolically inert. Consequently, the isotopic content of a feather reflects the bird’s diet—and by extension, the area where the feather developed—during the short period of time when the feather was grown. Shorebirds that breed in the Arctic and winter in South America, for example, complete their molt of flight feathers just before spring migration. Thus, isotopic ratios, or “signatures,” in the flight feathers of an adult bird captured during migration or on breeding grounds will correspond to those of the bird’s previous non-breeding area. The cooperative study recently initiated between the USGS and partners in other countries will evaluate (1) variation in isotopic signatures across the non-breeding range and (2) the use of these signatures to identify linkages to specific migration corridors and breeding areas. Google “environmental isotopes”, or go to Science News Daily or other reputable websites to find isotopes.