Name: Period: ______ Date: Learning Target: I can explain how
advertisement

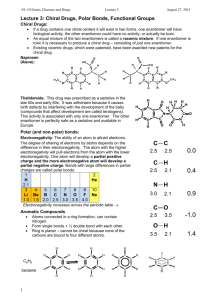
Name: ____________________________________________ Period: __________ Date: __________________ Learning Target: I can explain how atoms form covalent bonds. Criteria for Success: I can explain the difference between polar covalent and non-polar covalent bonding. I can classify a bond as either polar covalent or non-polar covalent based on electronegativity differences. I can determine the net polarity of a molecule. Polar Covalent and Non-Polar Covalent Bonding A. ______________________ bonding can be either _________________________ or ___________________________. 1. A ____________________________________ bond is a covalent bond in which the bonding electrons are shared ____________________ by the bonded atoms. a. Generally speaking, ____________________________________ bonds occur between atoms with electronegativity differences of _________________________________. 2. A _________________________________________ bond is a covalent bond in which the bonded atoms have an ______________________ attraction for the shared electrons. a. Generally speaking, __________________________________ bonds occur between atoms with electronegativity differences of _______________________. b. A ______________________ is used to represent this _____________________ sharing of electrons. A _____________________ is represented by an ________________ with the head pointing toward the ___________________ pole and a crossed tail pointing toward the __________________ pole. 3. Using VSEPR theory, we can determine if there is an overall ___________ polarity to the molecule and draw a single ___________________ to depict this. Periodic Table of Electronegativity Directions: Draw the Lewis structure for each molecule indicated below. Using the periodic table of electronegativity, determine if the bonds are polar. If the bonds are polar, indicate the direction of the dipole for each bond. Finally, using VSEPR theory and your understanding of molecular geometry, determine whether the molecule will have a net dipole (be a polar molecule overall). N2 1. Electronegativity Difference: ____________ 2. Are the individual bonds polar or non-polar 3. Molecular Geometry: ______________________ 4. Is the overall molecule polar or non-polar H2O 9. Electronegativity Difference: ____________ 10. Are the individual bonds polar or non-polar 11. Molecular Geometry: ______________________ 12. Is the overall molecule polar or non-polar CH4 17. Electronegativity Difference: ____________ 18. Are the individual bonds polar or non-polar 19. Molecular Geometry: ______________________ 20. Is the overall molecule polar or non-polar HCl 5. Electronegativity Difference: ____________ 6. Are the individual bonds polar or non-polar 7. Molecular Geometry: ______________________ 8. Is the overall molecule polar or non-polar CS2 13. Electronegativity Difference: ____________ 14. Are the individual bonds polar or non-polar 15. Molecular Geometry: ______________________ 16. Is the overall molecule polar or non-polar NH3 21. Electronegativity Difference: ____________ 22. Are the individual bonds polar or non-polar 23. Molecular Geometry: ______________________ 24. Is the overall molecule polar or non-polar



![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)