W1 buffer
advertisement

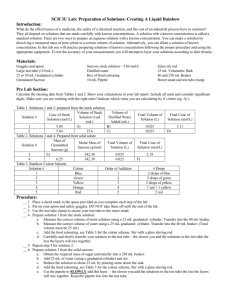
Separation of E.coli Inner and Outer Bacterial membrane Materials: W1 buffer (pH7.4) 20mM MOPS 30mM Na2SO4 5mM MgSO4 Suggested stocks: 10x stock solutions: 200mM MOPS, 300mM NaSO4 pH7.4 and 1M MgSO4. Or another buffer of your choice. X% Sucrose buffers: W1 buffer plus sucrose to 55% (691.9g), 50% (614.8g), 45% (541.3g), 40% (470.6g), 35% (402.9g), 30% (338.1g), 25% (275.9g). This is done w/w so therefore the weight plus MilliQ water to 1 litre (1000g). Protocol: Preparation of Sucrose Gradients Prepare sucrose gradients in 70ml Beckman centrifuge tubes in 10ml layers of 55,50,45,40,35,30,and 25 (pellet in 25%)% (W/W) sucrose in W1 buffer. Stocks are stored at in -20°C. 1. Pre cool Ti45 ultra centrifuge tubes on ice. 2. Swirl the buffers before use to ensure they are mixed. 3. Start with the most dense (55%) and put 5ml at the bottom of the Ti45 tube using a size 16/18 gauge needle and a 10ml syringe. 4. Gently trickle 10 ml of the 50% buffer down the side of the centrifuge tube, (do this very, very slowly) to make the next layer of the gradient. It will form an interface between the two layers. 5. Wipe needle between additions of sucrose. 6. Repeat the layering until 30% ends up at the top of the tube and 55% at the bottom. 7. Leave gradients for a minimum amount of time possible. They are ready to use. Keep on ice. 8. Layer the mixed membrane fraction on to the sucrose gradient (in 25% sucrose buffer). Use a thin needle to VERY slowly trickle the solution down the side of the tube. Do not disturb the sucrose gradient. Inner and outer membrane separation: 1. Keep the centrifuge and rotor at 4*C. 2. Load centrifuge (cooled rotor inside centrifuge chamber). Take care with tubes so gradients are not disturbed. 3. Set centrifuge: Speed: 38,000rpm Time: 16 hours (total 18hrs inc 2h breaking) Temp: 4°C Acceleration: 9 (slowest possible) Deceleration: no break, therefore it takes 2 hrs to stop. Fill in the log book.