The Effect of Temperature on Solubility
advertisement

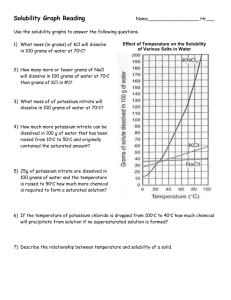
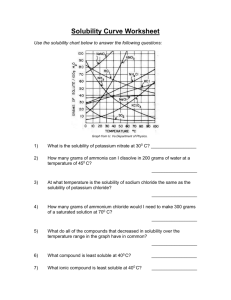
The Effect of Temperature on Solubility of Potassium Chloride In this activity, you are to determine the effect of temperature on the solubility of potassium chloride, KCl, in water. After collecting data at various temperatures, you will plot the data to produce a solubility curve showing how the solubility of potassium chloride varies with temperature. The graph you make can be used to predict the solubility at other temperatures. Procedure: 1. Obtain four test tubes containing potassium chloride solutions. Test tube A contains 2.50 grams of potassium chloride dissolved in 5.00 ml of solution. Test tube B contains 2.20 grams of potassium chloride dissolved in 5.00 ml of solution. Test tube C contains 1.80 grams of potassium chloride dissolved in 5.00 ml of solution. Test tube D contains 1.50 grams of potassium chloride dissolved in 5.00 ml of solution. 2. Fill the large beaker about ¾ full of water to use as a hot-water bath and begin heating it. 3. Place one of the test tubes in the hot-water bath. With a thermometer, stir the KCl solution until all the solid has dissolved. 4. Carefully hold the test tube up to a light. Watch for the very first signs of crystallization, and then immediately note the temperature. If crystallization begins too quickly, or you do not catch it the instant it begins, reheat the solution and try again. 5. Repeat steps 3 and 4 for all four test tubes. It may be necessary to use an ice bath to cause crystallization for some of the solutions. Simply place 3 or 4 pieces of ice in a beaker and fill half way with water. Data Table: Test Tube A B C D grams of KCl / 5.00 ml solution 2.50 2.20 1.80 1.50 Temperature at crystallization (°C) Molarity of solution Calculations and questions: 1. Calculate the molarity of each of the four solutions. Show your work. 2. Make a data table like the one shown above. 3. Make a graph of temperature on the x-axis and molarity on the y axis. 4. How are the solubility of potassium chloride and temperature related? 5 Find the solubility vs. temperature graph in your book (page 474). How is the solubility of most salts related to temperature? Are there any exceptions to this trend? Which substance has the least change in solubility? 5. Using the solubility vs temperature graph in your book determine how many grams of ammonium chloride can be dissolved in 100 grams of water at 70°C. 6. Use the solubility vs temperature graph in your book to determine how many grams of potassium nitrate can be dissolved in 300 grams of water at 30°C.