Build an Atom PhET Simulation Name PRELAB Atoms are made of
advertisement

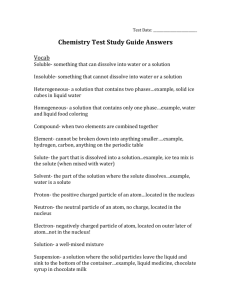
Build an Atom PhET Simulation Name _____________________ PRELAB Atoms are made of three subatomic particles; protons, neutrons, and electrons. Protons have a mass of ___________ amu and a charge of ___________. Neutrons have a mass of ___________ amu and a charge of ___________. Electrons have a mass of nearly___________ amu and a charge of ___________. Put the following subatomic particles in the model: 4 protons P P P P 5 neutrons 4 electrons N N E E N N N E E Circle which element this atom is on this periodic table below: The mass of this atom or ion is: a. 4 mass units I chose this answer because______________ b. 5 mass units ____________________________________________ c. 8 mass units ____________________________________________ d. 9 mass units ____________________________________________ e. 13 mass units Fill in the following with one of the following: protons, electrons, neutrons 1. I can change the type of atom by adding:_______________________________________ 2. I can change the charge of a specific atom by adding:______________________________ 3. I can change the mass of a specific atom by adding: _______________________________ Double Click on the button icon. Scroll down and select then click click on the reset all button. open the boxes called Net Charge and Mass number 3. Tryputting some protons into the nucleus of the atom (on the X) What happens to the: Mass number? ________________________________________________________________ Charge? _____________________________________________________________________ Does it stay on the X?_____________Does the symbol change on the periodic table?__________ Return protons to the bowl. 4. Try putting some neutrons into the nucleus of the atom (on the X) What happens to the: Mass number? ________________________________________________________________ Charge? ____________________________________________________________________ Does it stay on the X?_________Does the symbol change on the periodic table?______________ Return the neutrons to the bowl 5. Experiment by putting some 10 electrons into the nucleus of the atom (on the X). What happens to the: Mass number? _______________________________________________________________ Charge? ____________________________________________________________________ Does it stay on the X?_________Does the symbol change on the periodic table?______________ Return the electrons to the bowl starting with the ones on the inner level. What do you notice happening everytime an electron is removed from the first level? __________ ___________________________________________________________________________ 6. Put 3 protons into nucleus of the atom. Fill in the following: Name of atom:____________ atom or ion? ____________ net charge ____________ Decide how you will build a neutral atom that is stable. Practice making atoms using your ideas. Once you are able to do this several times on the simulation- starting with different numbers of protons- write out the steps of your building plan! steps to build a neutral atom starting with protons: 1. First I choose _______ protons and put them in the center (nucleus) of the atom. 2. 3. 4. *My stable atom: ____ mass ____protons ___ neutrons ___ electrons _______ name of atom? Analysis Questions 1. Ions are atoms of the same element with different numbers of __________________. 2. Isotopes are atoms of the same element with different numbers of __________________. 3. Adding or removing protons from an atom does what to the atom? _________________________________. 4. An atom with the same number of protons and electrons has a charge of _________. 5. Adding two electrons to a neutral atom produces an ion with a charge of _________. 6. An atom with six protons and five electrons would have a charge of _________. 7. What atom is created with nine protons, nine neutrons, and nine electrons? 8. Show the full symbol for the above atom in the box at the right 9. What does the upper-left number in the symbol represent? __________________ 10. What does the lower-left number in the symbol represent? __________________ The Game With remaining class time, play a few games. Who in your lab group can get the highest score? WINNER: _______________