L15-01346R1 supp
advertisement

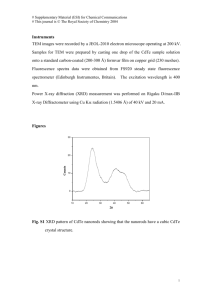
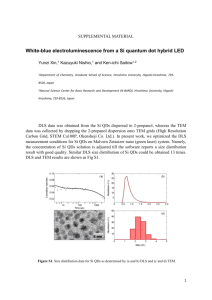
Supporting Information Fluorescent Silicon Nanoparticles Utilized As Stable Color Converters for White Light-Emitting Diodes Liu Yang,a) Yuan Liu,a) Yiling Zhong, Xiangxu Jiang, Bin Song, Xiaoyuan Ji, Yuanyuan Su, Liangsheng Liaob) and Yao Heb) Institute of Functional Nano & Soft Materials (FUNSOM) and Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu 215123, China. E-mail: lsliao@suda.edu.cn, yaohe@suda.edu.cn (Y. He) S1 Experiment details Synthesis of SiNPs: The SiNPs precursor solution was prepared by adding 100 mL of (3-aminopropyl)trimethoxysilane (97%, purchased from Sigma-Aldrich) to 400 mL N2-saturated aqueous solution dispersed with 18.6 g of trisodium citrate dihydrate (≥99.0%, purchased from Sinopharm Chemical Reagent Co., Ltd). The mixture was stirred for 10 min. For the investigation of the pH effect, analyticalgrade HCl (0.1 M) and NaOH (0.5 M) were added in the mixture to obtain the desired pH (pH = 4, 8, 10, 12). The pH value was monitored by Seven Multi pH meter (Mettler Toledo). The resultant precursor solution was transferred into the exclusive vitreous vessel with a volume of 30 mL. The SiNPs were prepared under 160 °C/15 min. After microwave irradiation, the SiNPs sample was removed when the temperature cooled to < 30 oC naturally. To exclude impurities influence, such as (3-aminopropyl)-trimethoxysilane molecules and trisodium citrate dihydrate in solution, the residual reagents were removed by dialysis (3 kDa). The microwave system NOVA used for synthesizing SiNPs was made by Preekem of Shanghai, China. The system operates at 2450 MHz frequency and works at 0-500W power. UV and PL measurement: UV−vis absorption spectra were recorded with a Perkin-Elmer lambda 750 UV−vis near-infrared spectrophotometer. PL measurements were performed using a HORIBA JOBTN YVON FLUOROMAX-4 spectrofluorimeter. Optical measurements were performed at room temperature under ambient air conditions. TEM and HRTEM: TEM and HRTEM samples were prepared by dispersing the sample onto carboncoated copper grids with the excess solvent evaporated. The TEM/HRTEM overview images were recorded using Philips CM 200 electron microscope operated at 200 kV. According to TEM characterizations, the particle size is decreased from ~3.7 nm to ~2.7 nm with pH value increases from 4 to 12. Previous S2 reports have shown that the particle size of the nanoparticles can be modulated by different reaction rate under the different pH value of the reaction solution. Typically, Aelion, Hench and co-workers separately report that the pH value of aqueous phase affects the electrolyte concentration on the hydrolysis of in the process of nanoparticles formation (Chem. Rev. 1990, 90, 33; J. Am. Chem. Soc. 1950, 72, 5705). Mou et al. report a method for tailoring the size of silica nanoparticles by adjusting the hydrolysis rate tetraethyl orthosilicate in reaction medium of different pH value, which leads to a series of silica nanoparticles with different sizes ranging from 30 to 280 nm (Small 2009, 5, 1408). These works are consistent with our work, e.g., in acidic conditions, the hydrolysis rate of the precursor molecules (i.e., (3-Aminopropyl)trimethoxysilane) is faster under microwave irradiation, thus leading to faster reaction rate. Hence, SiNPs with size ranging from 3.69 ± 0.57, 3.49 ± 0.39, 3.03 ± 0.42, and 2.71 ± 0.45 nm can be achieved in acidicto-basic conditions during the same time. DLS: Light-scattering analysis was performed using a DynaPro dynamic light scatterer (DLS), which was made by Malvern Corp, U.K. (ZEN3690). 1 mL SiNPs sample was transferred into an exclusive vitreous for DLS measurements. Experiment parameters were as follows: scan times: 100; dispersant: water; temperature: 25 °C; viscosity: 0.8872 cP; RI: 1.330; and dielectric constant: 78.5. Preparation of the LEDs: The configuration of our QD-LEDs was similar to that of the conventional LED. The blue InGAN LEDs (purchased from ShenZhen Dongrui Co., Ltd.) with the emission peak at 410 nm were used. The two components of the silicone precursor (Qsil218, ACC Silicones) were first mixed at a ratio of 10:1 (v/v). For the preparation of the white LEDs, the SiNPs prepared at pH value of 4 were milled to a fine powder. The silicone was mixed with the desired amount of SiNPs powder by agitating very slowly. Then, ~30 μL of the SiNPs and silicone mixture was dispensed on top of the bule LED and cured at 80 oC for 2h under air atmosphere. S3 EL, CRI, CIE and EQE: White-LED characteristics was measured by a constant current source (Keithley 2400s Source Meter) combined with a photometer (Photo Research PR 655 spectrophotometer) and an integrating sphere (LED300 + HAAS2000_V1_USB). The current density, driving voltage, brightness, power efficiency, EL spectra, CIE, CRI and EQE can be obtained simultaneously. Photostability Comparison of CdTe QDs and SiNPs: CdTe QDs were prepared based on our previous report (J. Phys. Chem. B 2006, 110, 13370). To test the stability of the SiNPs against high-temperature curing processes, both SiNPs and CdTe QDs were mixed with the silicone resin and cured at 80 oC for 2 h. To test the stability of the SiNPs against intense UV radiation, both SiNPs and CdTe QDs were dropcasted on the quartz substrate and exposed to 365 nm UV xenon lamp (ML-3500, MAXIMA) of 450 W intensity for 200 h. Photos of taken under exposure to 365 nm UV light. The PL intensities of the two samples were adjusted the same value. The samples were irradiated for different time intervals using a high-power xenon lamp (450W, 365 nm). FTIR and XRD: For FTIR measurements, KBr was pressed into a slice, onto which the SiNPs sample was dropped. The solvent in the sample was adequately evaporated by irradiation (>30 min) with a highpower incandescent lamp. FTIR spectra were recorded on a Bruker HYPERION FTIR spectrometer and cumulated 32 scans at a resolution of 4 cm-1. The powder X-ray diffraction (XRD) spectra were recorded on a Panalytical, Empyrean, X-ray diffractometer, operated at 40 mA and 40 kV. The SiNPs were placed on a zero-background sample holder made of monocrystal silicon plate. After evaporating the solvent, a film was formed and used for the measurement. The MTT assay of cell viability: ARPE (acute retinal pegment epitheliitis)-19 cells (in DMEM/F12 medium) were dispersed in 96-well plates (90 μL in each well containing 1×104 cells per well). 10 μL of S4 the SiNPs solution (OD = 1.15) was added to each well. Incubation was carried out for 3, 6, 12, 24, and 48 h in a humidified atmosphere at 37 °C with 5% CO2. The cytotoxicity of the SiNPs was evaluated by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Thiazolyl blue tetrazolium bromide (M5655); Sigma). The assay was based on the accumulation of dark blue formazan crystals inside living cells after exposure to MTT, which is well-established for assessment of cellular viability. The destruction of cell membranes by the addition of sodium dodecylsulfate (SDS) resulted in the liberation and solubilization of the crystals. The number of viable cells was thus directly proportional to the level of the initial formazan product created. The formazan concentration was finally quantified using a spectrophotometer by measuring the absorbance at 570 nm (ELISA reader). A linear relationship between cell number and optical density was established, thus allowing an accurate quantification of changes in the rate of cell proliferation. Additional data Figure S1 shows photostability of the SiNPs prepared at pH value of 4. Figure S2 shows enlarged TEM/HRTEM images of the SiNPs prepared at pH value of 4. Figure S3 shows FTIR and XRD spectra of the SiNPs prepared at pH value of 4. Figure S4 shows cytotoxicity assessment of the SiNPs prepared at pH value of 4. S5 Figure S1. Photos taken under exposure to 365 nm UV light (a-f): (a) SiNPs, initial. (b) SiNPs, after a silicone curing process. (c) SiNPs, UV exposure for 200 h. (d) CdTe QDs, initial. (e) CdTe QDs, after a silicone curing process. (f) CdTe QDs, UV exposure for 200h. (g) Photostability comparison of CdTe QDs and SiNPs. Photostability of the prepared SiNPs is also testified in our experiment, since color converters maintaining stable optical properties after packaging processes and long-term use are critically essential for practical LED applications. In our experiment, CdTe QDs (a typical kind of II-IV QDs) are compared as a control, and the stability of both samples (i.e., SiNPs and CdTe QDs) against intense UV radiation (365 nm, 450 W) and high-temperature curing processes is tested. As shown in Figure S1a-c, the SiNPs exhibit stable and bright fluorescence during high-temperature curing processes and long-term (e.g., 200 h) highpower UV irradiation. Such remarkable anti-bleaching property is attributed to the unique PL properties of SiNPs, as well discussed in previous reports.2 In marked contrast, the PL intensity of the CdTe QDs decreases significantly under the same experimental conditions, and their fluorescence is severely S6 quenched within 200-h intense UV irradiation (Figure S1d-f). The reason is that, when the CdTe QDs absorb high-energy photons continuously, the organic ligands coordinate on the QDs surface can be easily degraded or detached to generate surface defects that cause luminescence quenching or oxidation of the structure. Quantitative analysis of luminescent intensities is presented in Figure S1g. Typically, during 200-h continuous observation, the SiNPs maintained 90% of the initial luminescence, which is in sharp contrast to the CdTe QDs whose fluorescent intensity retained only 20% after 100 h (Figure S1g). These data provide convincing demonstration of ultrahigh photostability of the prepared SiNPs, facilitating construction of high-performance LED devices with robust stability. S7 Figure S2. TEM and HRTEM (inset) images of the SiNPs prepared at pH value of 4. Inset presents the enlarged HRTEM image of a single SiNP. The SiNPs show spherical structure with good monodispersibility. The well-resolved (220) lattice planes of ~0.19 nm spacing in the HRTEM image demonstrate excellent crystallinity of the as-prepared SiNPs. S8 Figure S3. (a) FTIR and (b) XRD spectra of the SiNPs prepared at pH value of 4. FTIR spectra features several distinct absorption peaks in the range of 1000−3500 cm−1. Typically, the sharp absorbance peak at ~1060 cm−1 is ascribed to the vibrational stretch of Si−O bonding. The strong absorbance at 1390−1440, 1580, and 3400 cm−1 are, respectively, assigned to the C−O and N −H bending vibrations and the N−H stretching vibration. XRD spectrum of the prepared SiNPs shows the characteristic (111), (220), and (311) reflections of diamond structure Si, confirming the nanocrystals are crystalline Si (J. Am. Chem. Soc. 2013, 135, 8350). S9 Figure S4. Cytotoxicity assessment of the SiNPs with the maximum emission wavelength of 545 nm. (a) Cell viability of ARPE-19 cells treated with the SiNPs for different incubation time. The cell viability was calculated as a percentage from the viability of the control (untreated) cells. The viability of the control cells was considered 100%. The results are means ± SD from three or four independent experiments. (b−f) Morphology of ARPE-19 cells after incubated with SiNPs for 3, 6, 12, 24 and 48 h, respectively. Typically, Figure S4a shows that the cell viability of SiNPs-treated ARPE-19 cells maintains above 90%, indicating SiNPs are noncytotoxic or low-cytotoxic due to favorable biocompatibility of silicon.2 As shown in Figure S4b-f, no obvious morphological change of ARPE-19 cells was observed in the presence of SiNPs, when the incubation time is extended to 48 h. These data suggest feeble cytotoxicity of the SiNPs to the cells, which is due to favorable biocompatibility of silicon and well consistent with previous reports.2 S10