Percentage yield of the product of a reaction
advertisement

Percentage yield of the product of a reaction
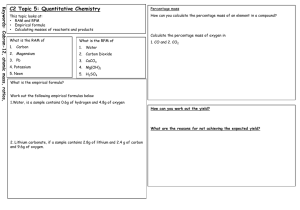
The % yield of a reaction is the percentage of the product obtained compared to the
theoretical maximum as calculated from the balanced equation.
In carrying out a chemical preparation, the aim is to work carefully and recover as much of
the desired reaction product as you can, and as pure as is possible and practicable.
However in any chemical process it is almost impossible to get 100% of the product because
of several reasons:
1. the reaction might not be 100% completed because it is reversible reaction and an
equilibrium is established.
2. You always get losses of product as it is separated from the reaction mixture by
filtration, distillation, crystallisation or whatever method is required.
3. Some of the reactants may react in another way to give a different product to the
one you want.
4. The aim is to work carefully and recover as much of the desired reaction product,
and as pure as is possible and practicable
% yield = actual amount of desired chemical obtained x 100 / maximum theoretical amount
formed
Example 14.2a.1
o
Magnesium metal dissolves in hydrochloric acid to form magnesium chloride.
o
Mg(s) + 2HCl(aq) ==> MgCl2(aq) + H2(g)
o
Atomic masses : Mg = 24 and Cl = 35.5, and formula mass MgCl2 = 24 + (2 x 35.5) =
95
o
(a) What is the maximum theoretical mass of magnesium chloride which can be
made from 12g of magnesium?
o
Reacting mass ratio calculation from the balanced equation:
1 Mg ==> 1 MgCl2, so 24g ==> 95g or 12g ==> 47.5g MgCl2
(b) If only 47.0g of purified magnesium chloride was obtained after crystallising the
salt from the solution, what is the % yield of the salt preparation?
% yield = actual amount obtained x 100 / maximum theoretical amount
possible
Example 14.2a.2
o
2.8g of iron was heated with excess sulphur to form iron sulphide.
o
Fe + S ==> FeS
o
The excess sulphur was dissolved in a solvent and the iron sulphide filtered off,
washed with clean solvent and dried.
o
If 4.1g of purified iron sulphide was finally obtained, what was the % yield of the
reaction?
o
1st a reacting mass calculation of the maximum amount of FeS that can be formed:
o
% yield = 47.0 x 100 / 47.5 = 98.9% (to 1dp)
Relative atomic/formula masses: Fe =56, FeS = 56 + 32 = 88
This means 56g Fe ==> 88g FeS, or by ratio, 2.8g Fe ==> 4.4g FeS
because 2.8 is 1/20th of 56, so theoretically you can get 1/20th of 88g of
FeS.
2nd the % yield calculation itself.
% yield = actual amount obtained x 100 / maximum theoretical amount
possible
% yield = 4.1 x 100 / 4.4 = 93.2% (to 1dp)
Example 14.2a.3
o
(a) Theoretically how much iron can be obtained from 1000 tonne of pure
haematite ore, formula Fe2O3 in a blast furnace?
o
If we assume the iron(III) oxide ore (haematite) is reduced by carbon monoxide, the
equation is:
o
Fe2O3(s) + 3CO(g) ==> 2Fe(l) + 3CO2(g)
o
(atomic masses: Fe = 56, O = 16)
o
For every Fe2O3 ==> 2Fe can be extracted, formula mass of ore = (2 x 56) + (3 x 56) =
160
o
Therefore reacting mass ratio is: 160 ==> 112 (from 2 x 56)
o
so, solving the ratio, 1000 ==> 112 / 160 = 700 tonne copper = max. can be
extracted
o
(b) If in reality, only 670 tonne of iron is produced what is the % yield of the overall
blast furnace process?
o
% yield = actual yield x 100 / theoretical yield
o
% yield = 670 x 100 / 700 = 95.7%
o
In other words, 4.3% of the iron is lost in waste e.g. in the slag.
Example 14.2a.4
o
Given the atomic masses: Mg = 24 and O = 16,
o
and the reaction between magnesium to form magnesium oxide is given by
the symbol equation
o
2Mg(s) + O2(g) ==> 2MgO(s)
o
(a) What mass of magnesium oxide can be made from 1g of magnesium?
o
2Mg ==> 2MgO
in terms of reacting masses (2 x 24) ==> {2 x (24 +16)}
so 48g Mg ==> 80g MgO (or 24g ==> 40g, its all the same)
therefore solving the ratio
1g Mg ==> w g MgO, using the ratio 48 : 80
w = 1 x 80 / 48 = 1.67g MgO
(b) Suppose the % yield in the reaction is 80%. That is only 80% of the magnesium
oxide formed is actually recovered as useful product. How much magnesium needs
to be burned to make 30g of magnesium oxide?
This is a bit tricky and needs to done in two stages and can be set out in
several ways.
Now 48g Mg ==> 80g MgO (or any ratio mentioned above)
so y g Mg ==> 30g MgO
y = 30 x 48 / 80 = 18g Mg
BUT you only get back 80% of the MgO formed,
so therefore you need to take more of the magnesium than theoretically
calculated above.
Therefore for practical purposes you need to take, NOT 18g Mg, BUT ...
... since you only get 80/100 ths of the product ...
... you need to use 100/80 ths of the reactants in the first place
therefore Mg needed = 18g x 100 / 80 = 22.5g Mg
CHECK: reacting mass calculation + % yield calculation CHECK:
22.5 Mg ==> z MgO, z = 22.5 x 80 / 48 = 37.5g MgO,
but you only get 80% of this,
so you actually get 37.5 x 80 / 100 = 30g
This means in principle that if you only get x% yield ...
... you need to take 100/x quantities of reactants to compensate
for the losses.
Below is an example of a more advanced level yield calculation