l3reactpaths - chemicalminds
advertisement

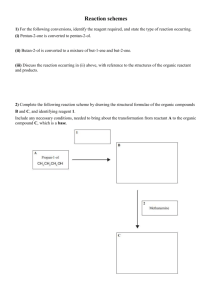
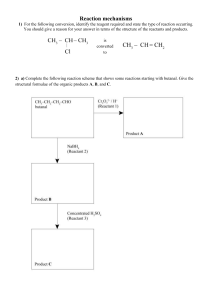
Reaction schemes 1) Propene can be reacted with water in the presence of acid to form a major product (A) and a minor product (B). • A is oxidised to form product C. • B is oxidised to form product D. • When D is reacted with SOCl2, it forms product E. • When D is reacted with alcohol B, it forms an ester G. • When D is reacted with alcohol A, it forms ester H, which is an isomer of G. • When E is reacted with alcoholic ammonia, it forms product F. • When E is reacted with water, it forms product D. 2) For the following conversions, identify the reagent required, and state the type of reaction occurring. (i) Pentan-2-one is converted to pentan-2-ol. (ii) Butan-2-ol is converted to a mixture of but-1-ene and but-2-ene. (iii) Discuss the reaction occurring in (ii) above, with reference to the structures of the organic reactant and products. 3) Complete the following reaction scheme by drawing the structural formulae of the organic compounds B and C, and identifying reagent 1. Include any necessary conditions, needed to bring about the transformation from reactant A to the organic compound C, which is a base. 4) When ammonia reacts with two products are formed. Complete the equation below by naming compounds or drawing the structure. 5) For the following conversion, identify the reagent required and state the type of reaction occurring. You should give a reason for your answer in terms of the structure of the reactants and products. 6) a) Complete the following reaction scheme that shows some reactions starting with butanal. Give the structural formulae of the organic products A, B,and C. b) Devise and complete the reaction scheme starting with butanone in place of butanal to show how butanone would react with the reactants 1–3. In your answer, you should: identify the products formed in each reaction step; state if no reaction occurs write the structural formula for each product formed, including major and minor products (if any). c) Compare and contrast the two reaction schemes. In your answer, justify the reasons for the similarities and the differences between these two schemes the products formed in each reaction. 7) Use the following information to answer this question. Compound W is a branched chain molecule with a molecular formula C4H10O. When Compound W is heated with excess acidified potassium dichromate it is readily oxidised to Compound X, which has acidic properties A substitution reaction occurs when Compound X is reacted with SOCl2. The molecular formula of Compound Y is C4H7OCl When Compound Y reacts with aminomethane, CH3–NH2, a substitution reaction occurs and Compound Z forms Determine the structural formulae of Compounds W, X, Y, and Z. Justify your answer by explaining how you arrived at these structures from the information given above. In your answer, you should: include other possible structural formulae you considered give your reasons for rejecting the other structural formulae. 8) The haloalkane 1-chlorobutane can be used to make butanamide. One of the intermediate products is a carboxylic acid. Show, using structural formulae, how this might be achieved in a number of reaction steps. Include all reagents 9) Alcohol A, (C4H10O) can react with Cr2O72– / H+ to give compound B which does not react with Tollens’ reagent. Compound A also reacts with SOCl2 to give a haloalkane C, which when reacted with alcoholic KOH, gives two products, D and E, which are not geometric isomers. When E reacts with H+ / H2O, A is the product. When D reacts with H+ / H2O, two products are formed, A and F. F can be oxidised to form butanoic acid. Give the structural formulae AND names for each of the compounds A to F. 10) Complete the following reaction scheme by naming and drawing the structural formula of each of the compounds A to F. Identify the reagents 1 to 4, including any necessary conditions, needed to bring about each transformation. 11) The haloalkane, 2-chloro-2-methylbutane, can be prepared by reacting 2-methylbutan-2-ol with concentrated HCl. i) Write an equation for this reaction using structural formulae ii) State the type of reaction occurring, and give a reason for your answer. 12) Identify the compound from these given: propanoyl chloride, 2-amino-3-methylbutane, pentanal, 4-chlorobutanoic acid that will react as described below, and draw the structural formula for each organic product formed. i) Elimination with alcoholic KOH ii) Oxidation with Fehling’s or Benedict’s solution iii) Substitution with aqueous KOH 13) Complete the reaction scheme by giving the formulae for reagents 1 to 4 and the structural formulae for the organic products A to D. 14) Propan-1-ol can be oxidised to produce two different products. Discuss how to carry out the oxidation of propan-1-ol in the laboratory to obtain two different organic products. The method may use two different samples of propan-1-ol to form each product. 15) Complete the following reaction scheme by giving the formulae for reagents 1 to 5 and the structural formulae for the THREE organic products. 16) 2-bromobutane reacts by substitution to form 2-butanol. However, if the reaction conditions are changed, an elimination reaction occurs. There are two possible products for the elimination reaction. Complete the following reaction scheme by indicating the reagents in the shaded boxes and the organic products in the other boxes for each of these reactions of 2-bromobutane. 17) Complete each of the equations below by writing the organic product in the blank boxes and the reagent needed in the shaded boxes. 18) For each reaction below, identify: (i) the type of reaction, (ii) the reagent required to carry out the reaction. 19) Complete the reaction scheme below by: a) i) identifying the three reagents ii) drawing the structures of compounds X and Y iii) naming organic compounds X and Y, and the compound with formula CH3CH2COCl b) Each of the parts (i) – (iii) below refers to one step in the reaction scheme. For each part, identify the type of reaction (from the given list) and use the reaction in that step to explain the term. addition, elimination, oxidation, polymerisation, substitution i) 2-chloropropane is converted to propene. ii) Compound X (the minor product) is converted to propan-1-ol. iii) Propan-1-ol is converted to propanoic acid. c) When HBr is reacted with propene, there are TWO possible products. Discuss how the compounds formed in this reaction scheme would vary if these two products were not separated before reagent 2 is added. 20) The following reactions involve the loss of water. Clearly show the structure of one major organic product of each of these reactions. Identify the type of reaction that is occurring in each case above: © 2015 http://www.chemicalminds.wikispaces.com NCEA questions and answers reproduced with permission from NZQA