National Institute for Clinical Excellence
advertisement
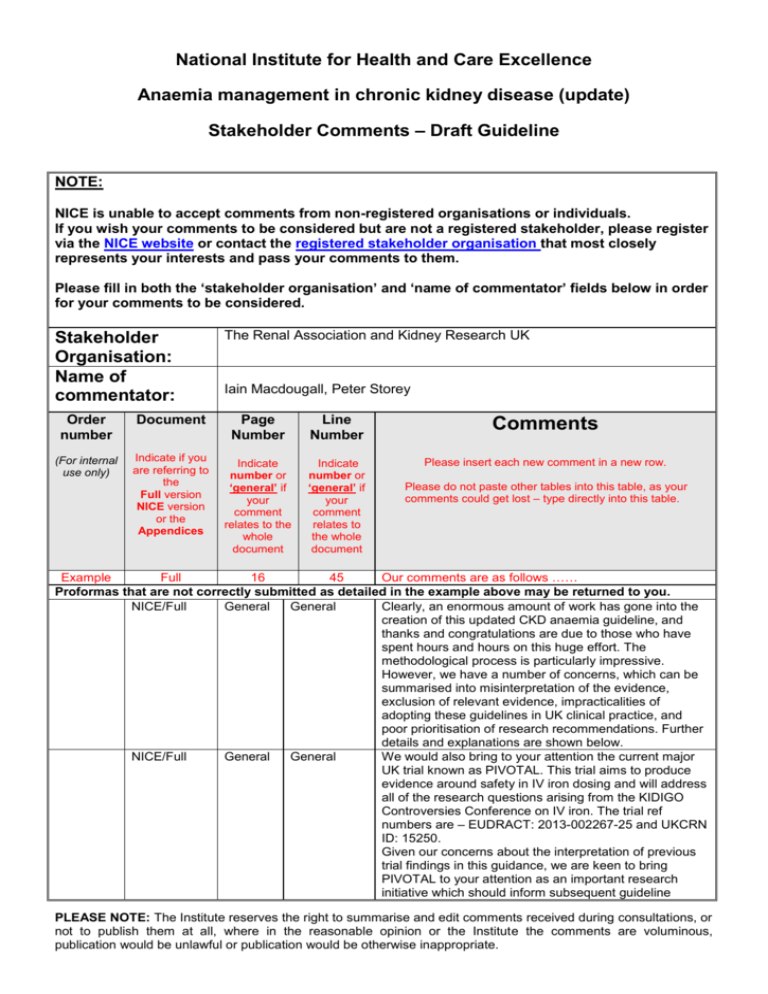
National Institute for Health and Care Excellence Anaemia management in chronic kidney disease (update) Stakeholder Comments – Draft Guideline NOTE: NICE is unable to accept comments from non-registered organisations or individuals. If you wish your comments to be considered but are not a registered stakeholder, please register via the NICE website or contact the registered stakeholder organisation that most closely represents your interests and pass your comments to them. Please fill in both the ‘stakeholder organisation’ and ‘name of commentator’ fields below in order for your comments to be considered. Stakeholder Organisation: Name of commentator: Order number Document (For internal use only) Indicate if you are referring to the Full version NICE version or the Appendices The Renal Association and Kidney Research UK Iain Macdougall, Peter Storey Page Number Line Number Comments Indicate number or ‘general’ if your comment relates to the whole document Indicate number or ‘general’ if your comment relates to the whole document Please insert each new comment in a new row. Please do not paste other tables into this table, as your comments could get lost – type directly into this table. Example Full 16 45 Our comments are as follows …… Proformas that are not correctly submitted as detailed in the example above may be returned to you. NICE/Full General General Clearly, an enormous amount of work has gone into the creation of this updated CKD anaemia guideline, and thanks and congratulations are due to those who have spent hours and hours on this huge effort. The methodological process is particularly impressive. However, we have a number of concerns, which can be summarised into misinterpretation of the evidence, exclusion of relevant evidence, impracticalities of adopting these guidelines in UK clinical practice, and poor prioritisation of research recommendations. Further details and explanations are shown below. NICE/Full General General We would also bring to your attention the current major UK trial known as PIVOTAL. This trial aims to produce evidence around safety in IV iron dosing and will address all of the research questions arising from the KIDIGO Controversies Conference on IV iron. The trial ref numbers are – EUDRACT: 2013-002267-25 and UKCRN ID: 15250. Given our concerns about the interpretation of previous trial findings in this guidance, we are keen to bring PIVOTAL to your attention as an important research initiative which should inform subsequent guideline PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. development. Publication of trial results is anticipated in 2018. NICE version 10 5 NICE version 10 General NICE version 10 17 NICE version NICE version 11 12 17 Paragraph 3 NICE version NICE version NICE version NICE version 13 14 21 21 1:1:3 1:1:4 1:3:18 Last paragraph We do not agree with the recommendation that iron status should be checked monthly in haemodialysis patients. We are aware that there is no evidence either way to inform this frequency of testing in this clinical context, and that this then becomes a matter of opinion. There are certainly some patients in whom monthly testing is sensible, but for a large majority, this frequency of testing is unnecessary. Most haemodialysis units in the UK currently test iron status every three months, and there would be logistic and economic implications in increasing this frequency to monthly, in the absence of any hard evidence that this is required. We are aware of the evidence to support measurement of hypochromic red cells as a marker of iron status, based largely on Tessitore’s study from 2001. However, this parameter is very prone to erroneous values if there is any delay in the blood sample being analysed. In Tessitore’s study, the blood samples were taken immediately from the dialysis unit to the lab, which was sited in close proximity to his dialysis unit. In many satellite dialysis unit patients, there is a considerable delay in the samples being analysed. Iain Macdougall advises that his experience with this parameter is that in a research study setting it performs excellently, but in real clinical life it is too unreliable and impractical (N.B. Iain’s view is that he isinversely conflicted with this opinion – he introduced measurement of this parameter to the global stage (Macdougall et al. Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ 1992; 304: 225-226), but is also very much aware of its limitations outside the research setting). Furthermore, the availability of analysers to perform this measurement is quite limited throughout the UK. Suggesting CHr as an alternative suffers from the same limitation, while other reticulocyte indices which are similar to CHr (and have since become available), such as Ret-He have not been validated properly in this setting. We do not fully agree with recommendation 1.1.4 that “transferrin saturation or serum ferritin should not be used alone to assess iron deficiency status in people with anaemia of CKD”. If a patient has a rock bottom serum ferritin level (e.g. 14 ug/l), then this patient is absolutely iron-deficient, and there is no value or need to check the transferrin saturation. As above, for comment 2. “When offering intravenous iron therapy to adults, consider high-dose low-frequency” — it is not clear whether this refers to both non-dialysis and haemodialysis patients. If it includes the latter, then we suggest there is no evidence to support this statement, and there is certainly a large observational study to suggest that this may be harmful (Brookhart et al, JASN 2013) As above, for comments 2 and 3. As above, for comment 4. As above, for comment 3. Not sure where the “600—1000 mg of iron” comes from. Although this does not seem an inappropriate amount, it PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. NICE version 22 1:3:19 NICE version NICE version 22 23 1:3:20 1:3:23 NICE version 23 1:3:25 NICE version NICE version NICE version 24 24 27—30 1:3:27 1:4:2 General – Research Recommend ations NICE version Full version 41—46 21 General 1:2:4 Full version Full version Full version Full version Full version Full version Full version Full version 60 61 62 65 65 66 67 92 5—18 3—4 9—21 25—30 34—36 20—21 10—18 18—20 Full version 93 36—38 may be better to say 500—1000 mg, since the iron preparations that may be used in this context come in 500 or 1000 mg vials. “Once percentage hypochromic red blood cells are less than 6%” should read “Once percentage hypochromic red blood cells is less than 6%”. As above, for comments 2 and 3. We do not agree with this recommendation. Patients who have end-stage renal failure and are on haemodialysis are both erythropoietin and iron deficient. We are not aware of any robust data showing that IV iron alone is successful in correcting the anaemia in this clinical scenario, and usually both ESA and iron supplementation are required. To recommend trying IV iron first is likely to result in patients suffering from anaemia for longer than they need to, with very little chance of success. We are not sure that oral iron should be offered to haemodialysis patients who choose not to have IV iron. The chances of this being of benefit are extremely slim due to no or negligible absorption from the gut, and the chances of side-effects are high. Clearly, patient choice is important in this context, but we do not think the health-care professional should be recommending this approach. As above, for comment 6. As above, for comment 2. While research into optimising anaemia management in acute illness and conservative care would undoubtedly be useful, this pales into insignificance in relation to the safety of intravenous iron. This is more relevant to a far larger patient group, and is relevant to all haemodialysis patients. Ferritin levels in this patient population in the UK are currently running at levels that have not been proven to be safe, and there is considerable laboratory and observational data that suggest that this could be harmful to a large majority of chronic haemodialysis patients. For this issue (which has been extensively commented on in the literature recently) not to have even been mentioned in the 2015 research recommendations seems to be a significant omission. As per comments above. In line with scientific evidence that has come to light over the last decade or so, upregulation of hepcidin activity should be mentioned as contributing to the pathogenesis of CKD anaemia. As above, for comments 2 and 3. As above, for comment 2. As above, for comments 2 and 3. As above, for comment 3. As above, for comment 10. As above, for comment 14. As above, for comments 2 and 3. This previous recommendation is still valid, is helpful, and should not be deleted. Low serum ferritin levels are 100% diagnostic of absolute iron deficiency, and will respond to effective iron replacement. We agree with the GDG that serum hepcidin has not shown any clinical value in the detection of iron insufficiency; we are not clear as to why the GDG felt that this was out of the scope of this guideline. If they are critically reviewing the evidence for detection of iron PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. Full version Full version Full version 108 109--117 214 20 top 14-15 Full version Full version 214 228 6:12:6 Table Full version 234 6.15.3.4 Full version 234 6.15.3.4 Full version 234 Full version 235 6.15.3.4, 5th last line 6.15.3.4 deficiency, then the paper by Tesssitore in 2010 (Nephrology Dialysis Transplantation 2010; 25: 39964002) provides stronger evidence (albeit negative) than any paper that supports the use of Ret He in the monitoring of iron status in people with CKD, and yet the GDG seem happy to make statements recommending the latter novel reticulocyte parameter. As above, for comment 3. As above, for comments 2 and 3. We do not agree with the statement “that lack of response to ESA (hypo-responsiveness) may be partly due to any discontinuation of iron”. It is now fairly clear that inflammatory cytokines directly suppress erythroid cell proliferation in the bone marrow, and IL-6-generated upregulation of hepcidin activity causes iron to be locked away in the reticuloendothelial system. We do not believe that discontinuation of iron therapy contributes meaningfully to ESA hyporesponsiveness. As above, for comment 17. “Macdougall” is mis-spelt. Also, the superscript ‘214’ is awkwardly split. “Very low quality evidence from one study214 (n=461) suggested that oral iron performed better compared with high-dose/low-frequency IV iron when considering numbers of people receiving blood transfusion(s) and numbers of people requiring initiation of ESA therapy”. We do not think there is any evidence from this study to support this statement (see Appendix for raw data), and there is also a methodological flaw in making this assertion. The study was neither designed nor powered to show this, and more importantly, patients randomised to the oral iron group were more likely to then be treated with intravenous iron if they remained anaemic. Many of these subjects responded quite well to IV iron and did not need to receive ESA therapy, making it appear (falsely) as if oral iron was keeping patients off ESA therapy. In contrast, patients randomised to intravenous iron moved on to ESA therapy, since they had already exhausted any benefit from iron management. This therefore introduced a bias in the oral iron group, rendering any such analysis meaningless. Thus, interpretation of the primary endpoint should include the full composite, and looking at the individual components, as has been attempted in the draft NICE guidance is scientifically flawed. “With regard to other important iron parameters, such as measures of SF and TSAT, moderate to low quality evidence suggested little clinically important difference between the IV iron therapy and oral iron therapy”. We do not consider the differences in serum ferritin achieved between the high-dose IV iron group and the oral iron group in the FIND-CKD study214 to be clinically insignificant (see Appendix figure). “the control group were observed” should read “the control group was observed”….. “Very low quality evidence from one study214 (n=461) suggested that low-dose/high-frequency IV iron was preferable when considering numbers of people requiring blood transfusion(s), but low quality evidence from the same study suggested that oral iron was preferable when considering numbers of people needing to initiate ESA therapy”. For the same reasons outlined in comment 34 PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. above, this analysis and statement is scientifically flawed. Full version 236 6.15.4.2 Full version 253 Full version Full version 254 260 Four lines above Table 92. Table 6.15.5.4 Full version Full version Full version Full version 262 270 272 280 6.15.6 6.15.7 Lines 30-31 Line 34 Full version Full version 284 285 11th last line Recommend ation 1 Full version Full version 286 296 Full version 296 7.5.6 Reference List Reference List “We searched for randomised trials comparing the effectiveness of different iron preparations given as either IV low-dose/high-frequency, IV high-dose/low-frequency or oral regimens in people receiving ESA”. This reference appears not to have been used: Macdougall et al. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anaemia in patients with CKD. Clin J Am Soc Nephrol 2014; 9: 705-712. “hyposensitive” should read hypersensitive”. “Macdougall” is mis-spelt. “All-cause mortality rates were lower in people receiving low-dose/high-frequency IV iron compared with oral iron, but this a highly uncertain finding based on low quality evidence214 (n=460). The FIND-CKD study was neither designed nor powered to examine mortality, thus we do not think that this statement is valid and may be a dangerous assumption. As above, for comment 14. As above, for comment 17. As above, for comment 2. We do not think in 2015 that aluminium toxicity should be considered as a cause of ESA resistance. For practical purposes, this complication in dialysis patients has now become extinct. “Mircera” is mis-spelt. We do not think in 2015 that aluminium toxicity should be considered as a cause of anaemia in ESRD. For practical purposes, this complication in dialysis patients has now become extinct. As above, for comment 17. For reference 1, the “Canadian Erythropoietin Study Group” should appear before the title of this paper. In quite a number of the references, the number of authors listed appears fairly random. Moreover, the use of ‘et al’ also seems fairly random, e.g. in reference 210 only the first two authors are included, with no ‘et al’, while many other references have more authors included with or without ‘et al’. Please add extra rows as needed. Please email this form to: AMCKD@nice.org.uk Closing date: 5pm 8th January 2015 PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. Appendix PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate. PLEASE NOTE: The Institute reserves the right to summarise and edit comments received during consultations, or not to publish them at all, where in the reasonable opinion or the Institute the comments are voluminous, publication would be unlawful or publication would be otherwise inappropriate.








