The Atom: Basic Building Block of all Matter
advertisement
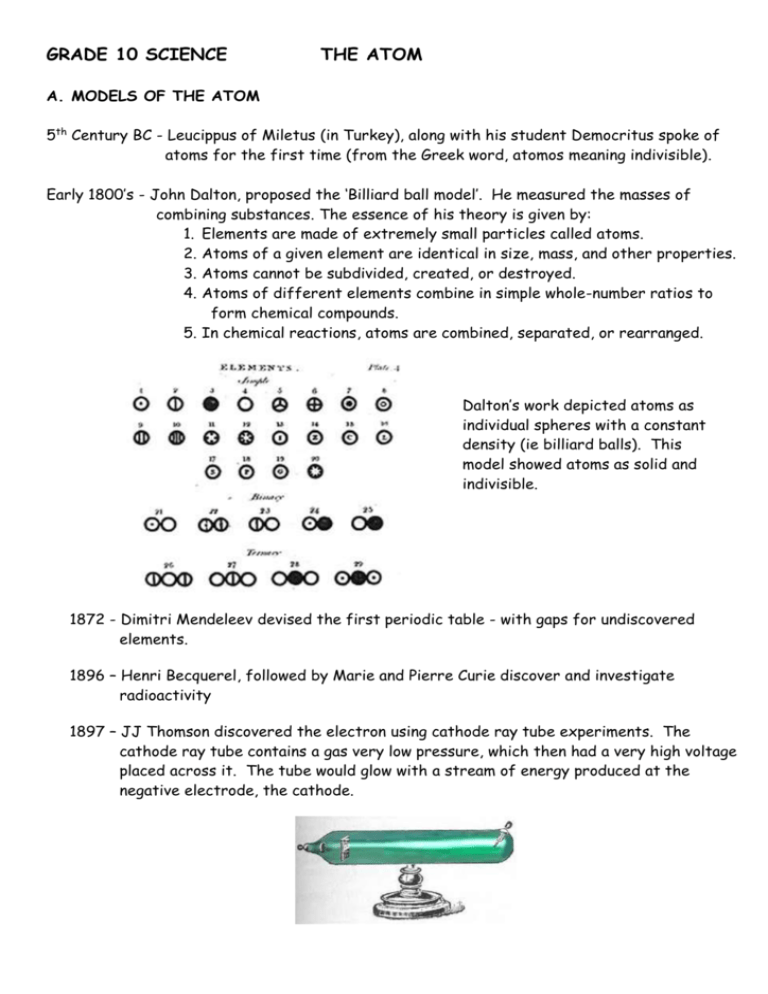
GRADE 10 SCIENCE THE ATOM A. MODELS OF THE ATOM 5th Century BC - Leucippus of Miletus (in Turkey), along with his student Democritus spoke of atoms for the first time (from the Greek word, atomos meaning indivisible). Early 1800’s - John Dalton, proposed the ‘Billiard ball model’. He measured the masses of combining substances. The essence of his theory is given by: 1. Elements are made of extremely small particles called atoms. 2. Atoms of a given element are identical in size, mass, and other properties. 3. Atoms cannot be subdivided, created, or destroyed. 4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged. Dalton’s work depicted atoms as individual spheres with a constant density (ie billiard balls). This model showed atoms as solid and indivisible. 1872 - Dimitri Mendeleev devised the first periodic table - with gaps for undiscovered elements. 1896 – Henri Becquerel, followed by Marie and Pierre Curie discover and investigate radioactivity 1897 – JJ Thomson discovered the electron using cathode ray tube experiments. The cathode ray tube contains a gas very low pressure, which then had a very high voltage placed across it. The tube would glow with a stream of energy produced at the negative electrode, the cathode. Thomson noted that the cathode rays were deflected by both electric and magnetic fields, deducing that the rays were a stream of negative particles of energy, called electrons. He then passed electric current through different gases and using different electrodes and found that the relationship of the charge to mass of the particles making up the rays was constant. He also likened the atom to a Plumpudding (similar to a fruit cake), which was a sphere that contained an equal number of positive and negative charges spread evenly throughout the sphere. 1911 – Ernest Rutherford – Gold foil experiment. Alpha (α-) particles (positive Helium nuclei) were shot at a very thin piece of gold foil. Most α- particles went straight through the foil, a few were deflected and even fewer bounced straight back. Conclusions: - the atom consists mainly of open space in which the electrons spin - at the centre of the atom is the nucleus that contains almost all the mass of the atom. 1913 - Niels Bohr produced the ‘Bohr Model’ of the atom - electrons are arranged in shells, or energy levels around the atom. He described: - electrons in atoms having their ‘ground state’ and occupying certain, fixed orbits or quantum levels. - an electron could be given energy and it would then become excited and move to a new orbit. - on losing that energy, light would be emitted, hence the different spectra for different gases. His model put scientists on the correct path, but it could only be used to explain the spectrum for hydrogen 1924 – Louis de Broglie – an electron has wave as well has particle properties 1927 – Werner Heisenberg’s Uncertainty Principle states that we cannot know both the position and momentum of an electron simultaneously. 1927 – Erwin Schrödinger produced the Schrödinger Wave Equation – used to mathematically calculate a region of space in which there is a high probability of finding an electron. 2 2 + V(r) (r) = E (r) 2m This is one form of the equation - each letter and symbol has a significance! 1932 – James Chadwick – discovered neutrons – each one having the same mass as a proton but no charge. B. ATOMIC MASS AND DIAMETER The size of an atom is incredibly small. - one atom of Ca - diameter of 197pm – 197 picometres or 197x10-12m. - mass of a typical calcium atom is 6.6x10-23g. Since the mass is so small, alternative ways of looking at mass are used: - atomic mass units ( a.m.u.) where 1 a.m.u. = 1.66 x 10-27kg - 1 hydrogen atom has a mass of 1 amu - relative atomic mass – one atom of carbon-12 ( 12 6 C ) was chosen as the standard, and assigned a mass of 12 (no units). The mass of all other atoms are assigned based on their mass relative to that of 1 atom of carbon-12. - 1 atom of hydrogen-1 has a mass 1 12 that of carbon-12 - relative atomic mass = 1 - 1 atom of magnesium-24 has a mass 2x that of carbon 12 - relative atomic mass = 24 C. STRUCTURE OF THE ATOM The atom consists of 3 sub-atomic particles Sub-Atomic Particles Proton Neutron Electron Relative mass Relative charge 1 1 0 +1 0 -1 An atom’s structure is summarised by: A Z X A = mass number = no of nucleons Z = atomic number = no of protons = no of electrons X = the symbol of the element. A – Z = no of neutrons D.ISOTOPES Definition: Isotopes are atoms of the same element, having the same atomic number but a different number of neutrons. Relative atomic mass of naturally occurring elements – this is often not a whole number (see periodic table). Each isotope in a sample contributes to the mass, so the percentage of each present is needed to determine the relative atomic mass. Worked Example: On analysis of a spectrograph, it is found that a sample of chlorine consists of 75% of the atoms having a mass of 35 and 25% of the atoms, a mass of 37. Calculate the relative atomic mass of chlorine. As we are working with percentages, we can assume that from every 100 atoms, 75 have a mass of 35 and 25 a mass of 37. The average is therefore: 35 × 75 + 37 × 25 = 35.5 100 E. ENERGY QUANTISATION and ELECTRON CONFIGURATION Electrons occupy different energy levels in an atom, each of which has a specific energy. As the energy of electrons is thus limited to a certain specific values, it is said to be quantised. - higher energy levels are further away from the nucleus - electrons in the ‘ground state’ can absorb specific amounts of energy and move to a higher energy level (‘excited’) - they can then drop back down to lower energy levels, thus releasing specific amounts of energy, which is given of as electromagnetic radiation of a specific colour. - quantisation of energy leads to a set of specific colours for each atom, hence atomic line spectra are observed - Valence electrons are the electrons found in the outermost energy level of an atom. - Core electrons are the electrons found in the innermost energy level of an atom. - The energy levels are represented by the principle quantum number, n, where n = 1, 2, 3, 4 etc - The maximum number of electrons in each energy level is given by 2n2. - Within each energy level, electrons occupy areas in space called atomic orbitals. We use two types, s-orbitals and p-orbitals. - Each orbital can accommodate 2 electrons. - within an energy level, the s-orbital has slightly lower energy than the p orbitals. - We only consider the orbitals shown in the diagram. The electrons are assigned to orbitals according to the Aufbau principle, following a number of rules: Hunds Rule: - every orbital is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin Pauli’s exclusion principle: - no two electrons in the orbital can have the same spin. Aufbau diagrams: 1,2,3,4 = energy levels = orbitals = electrons of opposite spin 4s 4s 3p 3p 3p 3s 2p 3s 2p Potential Energy 4s Potential Energy Potential Energy Examples: 3s 2p 2s 2s 2s 1s 1s 1s 2- 17 Cl N 7 16 S Another way of representing the electron distribution is using the spdf notation (where d and f refer to the orbitals of elements with atomic number greater than 20). Using the three examples above: Cl: 1s2 2s2 2p6 3s2 N: 1s2 2s2 2p3 S2-: 1s2 2s2 2p6 3s2 3p6 Shapes of orbitals The Schrödinger Wave Equation is used to calculate the outside surface of a region of space in which there is a high probability of finding an electron – we can thus visualise the shapes of the orbitals:








