NZFGW-Anthea-Blackburn
advertisement

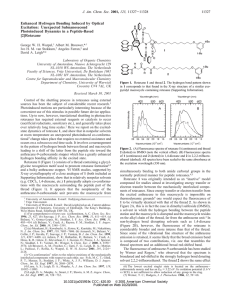
New Zealand Federation of Graduate Women 2012 Mid-Tenure Report Anthea Blackburn Biomimetics, that is, scientific endeavors inspired by nature, has shown itself to be an important tool in identifying solutions to the various challenges that scientists face today. Nature can be invoked to model analogous functions.1 One such natural phenomenon, which has been borrowed as an inspiration for some time in the development of new technologies, is electron transfer (eT). This process is commonly associated with photosynthesis in plants, as well as respiration in mitochondria, to name two examples. In all cases, eT is vital in the formation and transport of electrical energy between distant components within a cell, such that chemical energy can be generated for use as fuel.2,3 Solar cells, which carry out the harvesting of solar energy and its conversion into electrical energy,4 are one such technology that is inspired by photosynthetic eT. Similarly, biological eT has acted as the inspiration for the research proposed here – the development of a chemical system capable of undergoing eT as efficiently as that achieved biologically.5 Possibly the most impressive aspect of biological eT is the great distance over which it occurs. This directional long-range eT process is imperative in both natural and synthetic systems and has posed a challenge to scientists for some time now. Biologically, the protein cytochrome initiates this long-distance eT by converting photons from the sun into electrical energy in Photosystem II. It then expedites the transfer of these electrons to other parts of the photosynthetic cycle via quinone molecules in a multi-component process.6 Most importantly, in order for this eT to be controlled, efficient and directional, charge recombination must be prevented, so the electron donor and acceptor must be located as far apart as possible. Herein is the success of biological systems – a long-lived charge transfer state.7 In this research, we expect to take this process one step further and generate a one-component system that can facilitate long-range eT efficiently via a precisely aligned array of porphyrin moieties. In order to achieve efficient eT, I am exploiting the properties of inherently rigid and linear mechanically interlocked polyrotaxanes.8 A rotaxane consists of a thread, stoppered with bulky functional groups at either end (a dumbbell), and encircled by a controlled number of Figure 1: An oligorotaxane made up of a stoppered thread and m macrocycles. macrocycles; these molecules are named according to the convention [n]rotaxane, where n is the number of components that make up the molecule (Figure 1).9 In my research design, zinc (II) porphyrins have been attached to the macrocyclic portions of the oligorotaxane (Figure 2). It has been shown very recently that the parent oligorotaxanes can be easily self- assembled in quantitative yields.10,11 This protocol, known as clipping, relies on templation, provided by hydrogen-bonding and aromatic-aromatic interactions, to produce a homologous series of preordained oligorotaxanes easily and efficiently.12 Figure 2: A schematic of a porphyrinated oligorotaxane system, which facilitates eT via redox. Over the past seven months, I have focused on the synthesis of a model rotaxane complex, which has enabled me to determine the most successful and efficient strategy to attach the desired porphyrin moiety to the molecule (Figure 3). Despite some initial synthetic setbacks, the desired model rotaxane has been successfully synthesised and characterised. With this goal achieved, the electronic properties of the porphyrinic rotaxane can now be studied, that is, the molecule’s propensity to harvest light energy and transform it into electrical energy as an electron. In order for the transport of the Figure 3: Chemical structure of the porphyrinic rotaxane synthesized in this work. electron generated and the formation of the desired long-lived charge separated state, directionality needs to be introduced into the rotaxane. This will be achieved through the introduction of electron-donating and electron-accepting groups to either end of the molecule, as a way of introducing bias into the system, and thus directing the movement of the electron along the molecule. This is the basis of the photosynthetic process on which this research is focused. Outside of the lab, I have spent a large portion of my time volunteering at a local Chicago organisation, Family Matters. This is a not-for-profit company, which exists to build and strengthen the community through “programs that support personal growth and leadership.” In particular, I have been involved in the Community Tutoring component of Family Matters, which aims to encourage and support students in both their academic pursuits, as well as in their everyday lives. I have worked each week with a young Burmese refugee student, who arrived in Chicago from Thailand almost five years ago, where as a non-native English speaker, we spent a large portion of our time together working on her vocabulary, grammar and reading skills. One of the most rewarding aspects of this work is being able to make a difference in a student’s life and knowing that the hard work we carry out is appreciated – my tutee was honoured as the top student in her ESOL class this year! References 1. Benyus, J. Biomimicry: Innovation Inspired by Nature. William Morrow & Co.: New York, 1997. 2. Bassham, J., Benson, A., Calvin, M. "The Path of Carbon in Photosynthesis". J. Biol. Chem. 185: 781–787. 1950. 3. Krebs, A. “The History of the Tricarboxylic Acid Cycle”. Perspect. Biol. Med. 14: 154–170. 1970. 4. O’Regan, B., Grätzel, M. “A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films”. Nature. 353: 737–740. 1991. 5. Moore, R., Clark, W.D., Kingsley, R.S., Vodopich, D. Botany. Wm. C. Brown: New York, 1995. 6. Reedy, C.J., Gibney, B.R. “Heme Protein Assemblies”. Chem. Rev. 104: 617–650. 2004. 7. Karp, G. Cell and Molecular Biology (5th ed.). John Wiley & Sons: New Jersey, 2008. 8. Amabilino, D.B., Parsons, I.W., Stoddart, J.F. “Polyrotaxanes”. Trends in Polym. Sci. 2: 146– 152. 1995. 9. Silberberg, M.S. Principles of General Chemistry. McGraw-Hill: New York, 2007, 123–125. 10. Belowich, M.E., Valente, C., Stoddart, J.F. “Template-Directed Syntheses of Rigid Oligorotaxanes Under Thermodynamic Control”. Angew. Chem. Int. Ed. 49: 7208–7212. 2010. 11. Belowich, M.E., Valente, C., Smaldone, R.A., Friedman, D.C., Thiel, J., Cronin, L., Stoddart, J.F. “Positive Cooperativity in the Template-Directed Synthesis of Monodisperse Macromolecule”. J. Am. Chem. Soc. 134: 5243–5261. 2012. 12. Bravo, J.A., Raymo, F.M., Stoddart, J.F. “Molecular Meccano. Part 45. High Yielding Template-Directed Synthesis of [2]Rotaxanes”. Eur. J. Org. Chem. 11: 2565–2571. 1998.






![guise of bistable [2]rotaxanes in which the ring component can](http://s2.studylib.net/store/data/010343817_1-60384b1783ecd0bb22217894123718f2-300x300.png)
![[3]rotaxane 14 - Royal Society of Chemistry](http://s3.studylib.net/store/data/007797267_2-724bdc07a7bdf052cb4c149425f23d53-300x300.png)


