BMS Endo western blot practical 2013 Hout
advertisement

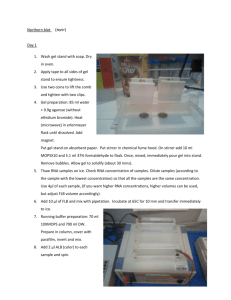
Part 1 Biomedical Sciences Opion 5: Endocrinology practical SDS-PAGE ELECTROPHORESIS AND WESTERN BLOTTING List of reagents / solutions: Tris-glycine running buffer TBS-T (Tris-buffered saline + 0.1% (v/v) Tween 20) Transfer buffer (running buffer + 20% (v/v) methanol) Methanol Coomassie blue stain and destain CN/DAB visualisation solution Pre-cast 10% SDS polyacrylamide gels Cell extract, negative and positive controls [These cell samples were prepared by resuspending a pelleted sample of cells in 100l PBS and then adding 25l of SDS loading buffer containing bromophenol blue, glycerol, β-mercaptoethanol and SDS (the dye makes the samples visible and acts as a front marker in the electrophoresis; the glycerol increases the density of the samples so that they can settle in the wells of the gel under the tank buffer)]. Sigma ‘Colourburst’ pre-stained protein standard markers (see p6 for details). Safety precautions: Unpolymerised polyacrylamide is a neurotoxin and the CN/DAB substrate is toxic, so you need to wear gloves during this practical. The electrophoresis and transfer apparatus uses high voltages. Please read the COSHH assessment on the end of this handbook. Do not touch the PVDF membrane with bare fingers as you will leave protein on it which might then cross-react with the antibody. 1 Part 1 Biomedical Sciences Opion 5: Endocrinology practical DAY 1 1. Running the gel to separate the proteins: Assemble the gel tank and add running buffer to the outer area of the tank (~5cm depth) and to the inner chamber (to the top). Each tank takes two gels, and the lower side of the glass plate faces the inside. Load marker and then your samples onto the gel (5ul of each). You will share a gel with another group, so use either lanes 2 (marker), 3 & 4 or 6 (marker), 7 & 8 Run gel at 100V through the stacking gel, then at 150V through the resolving gel, until the dye front reaches the bottom of the gel (~45 – 60 minutes). Keep an eye on the gel that it is still running during this time (bubbles should be visible coming off the bottom electrode). If the central buffer leaks out during the run, the circuit will be broken and the gel will stop running. When the gel is nearly finished: Pre-wet the piece of PVDF membrane in 100% methanol for about a minute, and then transfer to transfer buffer. Soak 4 pieces of filter paper in transfer buffer. 2. Transferring the protein to the PVDF membrane: It is important during the following procedure that everything is kept wet – you can pour a little transfer buffer onto the stack to keep it moist as you assemble it (but don’t drown it!). It is also essential that there are no air bubbles between any of the layers otherwise transfer will not occur. Use a glass rod as a roller to remove any bubbles. Diagrammatic representation of semi-dry transfer set-up. 1. Remove the cover from the semi-dry transfer apparatus. 2. Stack two layers of the pre-wetted filter paper onto the bottom plate, by individually layering them and ensuring that there are no air bubbles in between the sheets. 3. Add the piece of PDVF membrane, again ensuring there are no air bubbles. 2 Part 1 Biomedical Sciences Opion 5: Endocrinology practical 4. Remove the gel from the plate into some transfer buffer and cut off and discard the stacking gel. Put the resolving gel on top of the PVDF membrane, again ensuring there are no air bubbles. 5. Stack the other two layers of the pre-wetted filter paper on top of the gel, again by individually layering them and ensuring that there are no air bubbles between the sheets. 6. Put the top plate onto the apparatus and do up the screws (finger tight). 7. Transfer by running at 150V for 1 hour After transfer: 1. Disassemble the transfer apparatus, and remove the top layers of filter paper and the gel. 2. Make a mark with a pencil at the top of your marker lane to clearly indicate where the top of the resolving gel was. 3. Remove the membrane and cut it in half (using the prestained size markers as a guide) so that you separate your samples from those of the other group who shared your gel. 3. Western blotting protocol To prevent non-specific antibody binding, the membrane needs to be blocked with 5% (w/v) dried skimmed milk (‘blocking solution’) in TBS-T overnight. 1. Make up 5 mls of 5% (w/v) dried skimmed milk in TBS-T. 2. Place the membrane into a 15 ml plastic tube with the pencil mark facing the inside of the tube. Label the tube clearly with your name. 3. Add the 5 mls of the blocking solution, do up the lid tight and place on the tube roller to block overnight. DAY 2 4. Make up 2 mls of 5% (w/v) dried skimmed milk in TBS-T. 5. Discard the blocking solution and add the 2 mls of 5% (w/v) dried skimmed milk in TBS-T. Ask a demonstrator to add 2 μl of the anti-X HRP-conjugated antibody to your tube (to give a 1:1000 final antibody dilution). 6. Return the tube to the roller for 1 hour. 7. After 1 hour, tip off the antibody solution and wash the membrane three times for 5 minutes each with 5mls of TBS-T (i.e. 15 minutes washing in total). Put the tube back on the tube roller for each wash. 3 Part 1 Biomedical Sciences Opion 5: Endocrinology practical 4. CN/DAB staining 1. Put the membrane pencil mark side up into a large weigh boat. 2. Cover the membrane with 10 mls of the CN/DAB staining solution. 3. Gently agitate as the colour develops. 4. When developed, pour off the CD/DAB staining solution (back into a beaker, not down the sink) and rinse the membrane with distilled water. 5. Take a photo of your membrane. 6. Measure the distances that the bands have run from the top of the resolving gel and record this data so that you can draw the standard curve. 7. Write your names or similar clearly on the membrane so you can recognise it after Coomassie staining 5. Coomassie blue protein staining To show that there is protein on the membrane we will stain the membrane for total protein with Coomassie blue using the method below. 1. Pour on enough Coomassie blue to cover the membrane (~ 10 mls) and gently agitate for 10 minutes, then remove the Coomassie blue stain (return it to the original bottle) 2. Place in water (~ 10mls) for 5 minutes with agitation, then discard the water. 3. Add fresh water and leave. 4. We will dry the membrane on tissue paper and email you a photograph. 4 Part 1 Biomedical Sciences Opion 5: Endocrinology practical INFORMATION ON THE SIZE MARKERS THAT YOU USED (taken from http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Bulletin/c1992bul.Par.0001.File.tmp/c1992 bul.pdf) Apparent Molecular Masses (kDa) of ColorBurst Marker Proteins Band Color Violet Pink Blue Pink Orange Blue Pink Blue 5 Tris-Glycine gel 220 100 60 45 30 20 12 8 Part 1 Biomedical Sciences Opion 5: Endocrinology practical 6