Northern blot (יפעת) Day 1 Wash gel stand with soap. Dry in oven
advertisement

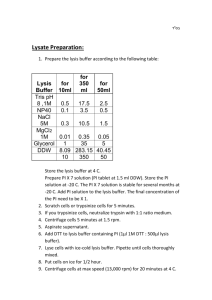
Northern blot )(יפעת Day 1 1. Wash gel stand with soap. Dry in oven. 2. Apply tape to all sides of gel stand to ensure tightness. 3. Use two coins to lift the comb and tighten with two clips. 4. Gel preparation: 85 ml water + 0.9g agarose (without ethidium bromide). Heat (microwave) in erlenmeyer flask until dissolved. Add magnet. Put gel stand on absorbent paper. Put stirrer in chemical fume hood. On stirrer add 10 ml MOPSX10 and 5.1 ml 37% formaldehyde to flask. Once, mixed, immediately pour gel into stand. Remove bubbles. Allow gel to solidify (about 30 mins). 5. Thaw RNA samples on ice. Check RNA concentration of samples. Dilute samples (according to the sample with the lowest concentration) so that all the samples are the same concentration. Use 4µl of each sample, (if you want higher RNA concentrations, higher volumes can be used, but adjust FLB volume accordingly). 6. Add 10 µl of FLB and mix with pipetation. Incubate at 65C for 10 min and transfer immediately to ice. 7. Running buffer preparation: 70 ml 10XMOPS and 700 ml DW. Prepare in column, cover with parafilm, invert and mix. 8. Add 2 µl ALB (color) to each sample and spin. 9. Remove tape and ruler from gel and set in bath. Make sure to cut upper right corner of the gel in order to know order of samples. Pour buffer until it covers gel. Put red plastic under gel. Load samples (begin from 2nd well if possible). 10. Hook black (negative) cable to top left of gel stand, and red (positive) cable to lower left of stand. On device: hook red to red and black to black. 11. Run gel: 100V 10-15 min 65V 30-40 min. 12. When samples reach center of gel, change direction of gel and switch cables so that liquid and salts are running in the opposite direction but direction of gel stays the same. Run 1 hr, or until sample coloring reaches 1-2cm before the end. 13. Membrane preparation: Cut 1 whatman paper to 35X12.5 cm (“large”), 6 whatman papers slightly larger than the gel size (usually 10 cm length X 12 cm) (“small”) and 1 nylon membrane also slightly larger than gel size. Cut one corner of membrane. Cut 4 long pieces of parafilm. 14. Buffer preparation: 200 ml SSCX20 and 200 ml water. 15. Pour buffer into a tip box, and pour some of the buffer into the stand. At each step, wet the whatman paper, the membrane and the gel by dipping in tip box, then place on center of stand, and use a glass tube to roll on top and remove air bubbles. Start with large whatman paper, then 3 pieces of small whatman paper, then the gel, then the membrane (make sure to align cut corners of gel and membrane) and then another 3 pieces of small whatman paper. 16. Pour equal amounts of buffer to both sides of the stand. 17. After adding buffer, stick parafilm on all 4 sides between plastic and whatman paper to prevent leaks. 18. Place (at least) 5 cm thick tissue glass pink book on top. Balance. 19. Add remaining buffer to sides and leave O/N. Day 2 1. Turn whatman+gel+membrane over. With a pen, mark wells on front side of membrane (the side which was in contact with the gel). Note that samples on membrane will appear in opposite direction from that loaded on gel! Remove membrane and dry lightly. 2. Place membrane(on a nylon surface) in UV light (100%) for 3-4 min (fixation). 3. Put in “whatman envelope” and dry at 80C for 30 min. Possible to keep for as long as needed. 4. Hybridization (start at about 12:00): Preheat hybridization shaker and bath to 68C. 5. Incubate 50 ml hybridization buffer in 68C bath. 6. Wash 1 glass tube and cap with soap for the membrane +1 for balance. Dry 10 min in 80C oven. 7. Add 50 ml warmed hybridization buffer to the glass tube and 50 ml water to the balance bottle. 8. Balance tubes and shake 1 hr at 68C. 9. Incubate 50 ml hybridization buffer + probe in 68C bath. 10. After 1 hr pour out liquid and add probe. Incubate in 68C shaker O/N. 11. DO NOT discard the probe! It can be reused. Keep tube at -20C and refill with probe when finished using it. Day 3 1. Put buffer and probe back in tube for re-use. 2. Incubate 50ml each at 68C until warmed: SSCX2 0.1% SDS and SSCX0.1 0.1% SDS buffers. 3. Perform 3 wash steps in hybridization bottle in shaker: Wash 1: SSCX2 0.1% SDS – 15 min. Wash 2+3: SSCX0.1 0.1% SDS– 30 min each. 4. Wash twice in a box on shaker with tween + maleate acid X1 – 1 min per wash, room temp. 5. Wash twice in a box on shaker with maleate acid X1 – 1 min per wash, room temp. 6. Blocking: 50 ml blocking buffer, incubate 1 hr in a box on shaker, room temp. 7. Centrifuge first Ab (AP - anti-Dig, refrigerated) 10,000 RPM, 5 min. 8. Add 2 µl of Ab to blocking buffer in box and incubate 1 hr on shaker, room temp. 9. Wash twice in a box on shaker with tween + maleate acid X1 – 5 min per wash. 10. Wash twice in a box on shaker with maleate acid X1 – 15 min per wash. 11. Prepare 50 ml fresh detection buffer and wash 5 min (keep 1 ml aside per membrane). 12. Place membrane in nylon and seal ) (לאטום3 sides, as close as possible to the membrane. Use sealer at temp. 2-3. 13. Add 10 µl second Ab (CSPD, refrigerated) to 1 ml detection buffer, add to nylon and seal. Use “rolling pin” “ ”מערוךto distribute liquid on top of membrane for 5 min. Incubate for an additional 5 min. 14. Cut one side of the nylon to remove ALL liquid then re-seal the nylon incubate sealed membrane at least 15 min at 37C. At this point it is possible to store at room temp. 15. Development: 30 min (or more, depending on probe) in dark room in a cassette with film (membrane first and film on top), then in a box with Developer water fixer water (1 min each). A weak expression in development needs longer exposure to film. Solutions MOPSX10 Sodium acetate 4.1g EDTA 3.72g MOPS 41.86g dH2O 800 ml Final concentrations: sodium acetate 50mM, EDTA 10mM, MOPS 0.2M. Adjust pH to 7 using NaOH 10M. Add dH2O to reach 1L. Autoclave. MOPS is sensitive to light. Wrap with silver foil. SSCX20 pH = 7-8 Tris sodium citrate 88.3g NaCl 175.39g dH2O 800 ml Check pH. Add dH2O to reach 1L. Final concentrations: Tris sodium citrate 0.3M, NaCl 3M. Autoclave. Agarose loading buffer - ALB Prepare large amount, aliquot and store at -20C. 50% glycerol 50% bromophenol blue FLP Prepare large amount, aliquot and store at -20C. Per eppendorf: MOPSX10 0.1 ml In fume hood add: Formaldehyde 0.3 ml Formamide 1 ml SSCX2 + SDS 0.1% 445 ml autoclaved DW 50 ml SSC 20 5 ml SDS 10% SSC 0.1% + SDS 0.1% 492.5 ml autoclaved DW 2.5 ml SSC 20 5 ml SDS 10% MaleateX5 pH 7.5 Maleic acid 58g NaCl 43.85g dH2O 700 ml Adjust pH with NaOH pellets. Add dH2O to reach 1L. Autoclave. Maleate X1 400 ml autoclaved DW 100 ml maleateX5 maleateX1 + tween 400 ml autoclaved DW 100 ml maleateX5 1.5 gr TweenX20 (weigh using cut tip). Make sure dissolved thoroughly. Blocking buffer 10 ml blocking 40 ml maleateX1 Detection buffer (prepare half) 88 ml autoclaved DW 10 ml Tris pH=9.5 1M 2 ml NaCl 5M Blocking solution 50g Casein 500 ml Maleate acid X1 Mix, microwave 1 min Mix, autoclave Prepare 500 ml in 1 L bottle Hybridization buffer 100 ml Blocking solution 125 ml SSCX20 1 ml SDS 10% 5 ml lauroysarcosil 10% 250 ml Formamide 19 ml autoclaved DW