homepage/sscott/file/Study%20Guide%20Unit%20III - Parkway C-2
advertisement

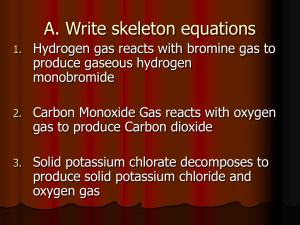
Study Guide Unit III – Chapter 11 Due Friday 11-09-12 (Blue) or 11-12-12 (Red) Main Topics: Chemical Reactions Balancing, Completing, and Identifying) Chapter 11 (p. 320 – 335) Know the four signals of a chemical reaction. Be able to use a solubility table (know the terms soluble, dissociates, aqueous and insoluble, precipitates, solid) Be able to use the reactivity series of metals table – and predict if a single replacement reaction will occur. Other Related Topics – that students need to remember and understand. Electronegativity (found in chapter 6) Ionic Bonding (Bonding Information p. 187 -193, Naming and formula writing p. 253 -266) Acids (Naming and formula writing p. 271 - 273) Diatomic Molecules (Chart on p. 222 – where two atoms bonded together when an element – H2, N2, O2, VIIA group F2, Cl2, Br2, I2) The test will be divided into sections Section I - Multiple choice section over any and all topics (about 15 questions) Section II – Balancing and Identifying the Type of Chemical Reaction (see summary on p. 338-339 – must know all five types) Section III - Includes: *Writing the reactants and products for a chemical reaction, when given the equation in words. *Writing the reactants and products for a balanced chemical equation, when given the reactants as words. *Finishing the product side of an equation and balancing it, when given the reactants as formulas. Section IV - Laboratory skills and knowledge – Students will go to the back of the room and perform a mini experiment by writing down the qualitative observations of the reactants before the mixing, during the mixing, and after the mixing. Then each student will have to decide if what is observed is a physical or chemical reaction and defend the choice. Students must know the four signals of a chemical reaction. Be able to tell what gas is released by testing it with a wooden splint (lit or glowing). Review questions You may write on this paper, but answers should be adequate. 1. What is the Law of Conservation of Mass (Matter) and why is it important to chemist. 2. List the four signals of a chemical reaction: 3. 4. 5. Compounds and/or elements before the arrow (to the left) are called __________ Compounds and/or elements after the arrow (to the right) are called __________ Which elements are diatomic in nature: How is an ionic compound different from an acid compound? 6. What are the rules for naming acids? 7. What does each symbol mean: + → ∆ ─ Pt → (s) (l) (g) (aq) What is a catalyst? 8. Which element has the highest electronegativity value? Which group of elements does not have electronegativity value? Explain the trend for electronegativity for a group (from top to bottom) and for a period (left to right). 9. Know the difference between a Cation (+) and an Anion (-). Be sure to know what happens to the charge of an atom, when the electrons are lost or gained respectively. Name O2 ___________________________________ HClO ___________________________________ Fe(SO4) ___________________________________ I2 ___________________________________ Al2(CO3)3 ___________________________________ Na(C2H3O2) _________________________________ H2SO4 ___________________________________ CO2 ___________________________________ K2CrO4 ___________________________________ Ag(NO3) ___________________________________ ZnO ___________________________________ H 2O ___________________________________ 10. Then you will also need to be able to write the formula from the name! 11. Thinking Formula Silver hydroxide ________________ ______________ Nitrous Acid ________________ ______________ Carbonic acid ________________ ______________ Carbon Dioxide ________________ ______________ Hydrochloric acid ________________ ______________ Calcium carbonate ________________ ______________ Lithium chlorate ________________ ______________ Hydrogen gas ________________ ______________ Lead (II) phosphate ________________ ______________ Barium sulfate ________________ ______________ 12. List all of the compounds from question #10 and #11 that would dissociate in water? [Hint use the solubility rules/table]: 13. 14. a. b. c. d. e. f. From the pairs given below choose and circle the metal that is more reactive. [Hint use the reactivity of metals table (back of periodic table or p. 333)] a. Li or Cu d. Al or Zn b. Ag or Cu e. Pb or Ca c. Mg or K f. Hydrogen (H2) or Cu Balance the following equations, using coefficients. Be sure to use the reaction rules, solubility rules, and activity series to develop a more thorough understanding. Also identify the type of chemical reaction it equation represents: combination, decomposition, single replacement, double replacement, or combustion. ___ H2O2 ——→ ____ H2O + ____ O2 type: ___________________ ___ Zn + ___ H3(PO4) ——→ ___ Zn3(PO4)2 + ___ H2 type:____________________ ___ Ag(NO3) + ___ PbI2 ——→ ____ Pb(NO3)2 + ___ AgI type: ________________ ___Al + ___ O2 ——→ ___ Al2O3 type: ___________________ ___Ca(OH)2 + ___HCl ——→ ___CaCl2 + __H(OH) (actually H2O) type: __________________ ___ Na2O + ___ H2O ——→ ___ Na(OH) type: _________________ Practice Chemical Equations (finishing, balancing and identifying) 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. Al(s) + O2 (g) __________ Mg + Ca(NO3)2 ________ + ________ Ca(s) + Mg(NO3)2(aq) ________ + ________ Cu(NO3)2 (aq) + Na2S(aq) _______ + ________ N2O3 + H 2O ________ K2CO3(s) ___________ + __________ Zn (s) + HCl (aq) _________ + ________ Al2(SO4)3(aq) + Ca(OH)2(aq) _________ + _______ C12H22O11 (s) + O2(g) ________ + _______ H2O2 ----Fe3+ --- ________ + _________ NH3 (g) __________ + __________ NaCl(aq) + K(NO3)(aq) ________ + _______ Type: _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ _________________ ________________ _________________ __________________ Double Check – for questions 15 – 26 were there some combinations that would be No Reaction? [Hint use the Solubility Table and Activity Series of Metals Table and Reactions Rules] Word Equations: Write the reactants and the products and then balance, also list the type of reaction that each is: 27. Solid Copper, plus Oxygen gas in a flame yields – 28. Zinc metal and Sulfuric Acid yields – 29. Magnesium nitrate solution and Sodium hydroxide solution placed together in a test tube yields – 30. Copper (II) Carbonate is heated and yields – 31. Methane from the Bunsen burner is reacted with oxygen in the air and yields 32. Lithium metal and water yields – 33. Hydrochloric acid and aluminum hydroxide yields – 34. Sulfur dioxide gas (SO2) and water yields – 35. Copper Hydroxide is heated and yields – 36. Burning sugar (C12H22O11) produces – 37. Calcium nitrate solution is mixed with sodium phosphate solution and yields - 38. Explain how to test for each of the following gases: Hydrogen (H2): Oxygen (O2): Carbon Dioxide (CO2): T The multiple-choice questions will require student to use conceptual knowledge to choose the best answer. It is especially important that the students be able to explain why something is the way it is. 39. Which one of the following equations is properly/correctly balanced? a. NH4NO3 2H2O + N2 b. CH3CHO + 3O2 2CO2 + 2H2O c. Sn + 4HNO3 SnO2 + 4NO2 + 2H2O d. 2Na2SO4 + 3Bi(NO3)3 Bi2(SO4)3 + 9NaNO3 e. Na2CO3 + 2H2SO4 Na2SO4 + 2H2O + CO2 40. Which element has the highest electronegativity? a. fluorine c. helium b. hydrogen d. neon 41. All of the following elements exist as diatomic molecules except a. hydrogen c. helium b. fluorine d. nitrogen 42. A double replacement reaction takes place when aqueous K 2(SO4) reacts with aqueous Pb(NO3)2. You would expect one of the products of this reaction to be: a. K 2S c. Pb(SO4) b. KPb d. K2NO3 43. The following reaction is balanced Sn + 4HNO3 → SnO2 + 4NO2 + 2H2O a. True b. false What kind of reaction is 2H2 + O2 → 2H2O? a. decomposition c. b. single-replacement d. combustion combination 44. 45. In all chemical and physical changes the atoms are a. lost c. gained b. not changed d. rearranged or spread apart but can be accounted for 46. Correctly balance the equation below and then draw the way the particles are arranged in the provided beakers. NaI (aq) + Pb(NO3)2 (aq) + 47. PbI2 (s) + Na(NO3) (aq) Correctly balance the equation below and then draw the way the particles are arranged in the provided beakers. Mg (s) + + HCl (aq) MgCl2 (aq) + H2 (g)